Published on: October 26, 2022
Written by Jonas Frank / Fact-checked by Nova Scarlett
Batteries are devices that store and release electrical energy. They have many uses, including powering electronic devices like cell phones and laptops. Batteries contain a number of chemical components, one of which is an electrolyte.
An electrolyte is a substance that conducts electricity when dissolved in water or another solvent. In a battery, the electrolyte facilitates the flow of electrons between the positive and negative electrodes.
The electrolytes in batteries are usually acids, bases, or salts. When the battery is being used, electrons flow from the negative electrode to the positive electrode through the electrolyte. This process creates electricity that powers the device connected to the battery.
The type of electrolyte used in a battery depends on the type of battery it is. For example, lead-acid batteries use sulfuric acid as their electrolyte while nickel-cadmium batteries use potassium hydroxide. Lithium-ion batteries, which are commonly used in cell phones and laptops, use a variety of different electrolytes depending on their application.
The concentration of the electrolyte also plays a role in how well the battery management system works. If the concentration is too low, there will not be enough ions to carry electrons between electrodes and power will be diminished. On the other hand, if the concentration is too high, it can cause corrosion within the battery which will shorten its lifespan.
An electrolyte is a substance that helps to conduct electricity. In a battery, the electrolyte helps to move electrons from one electrode to another. This process is what creates an electric current and allows a battery to power devices.
What is an Electrolyte in a Battery?
An electrolyte is a substance that produces an electrically conducting solution when dissolved in water. The solutions are called electrolytes. Electrolytes are present in all batteries, whether they are lead-acid, nickel-cadmium, nickel-metal hydride, or lithium-ion.
They are also present in fuel cells. The electrolyte consists of positive and negative ions which are attracted to the opposite electrodes (anode and cathode) in the battery. When the battery is being charged, the ions flow from one electrode to the other through the electrolyte.
This creates an electrical current. When the battery is being discharged, the process is reversed and electrons flow from one electrode to the other through an external circuit creating an electrical current.
What is an Electrolyte in a Battery Brainly?
An electrolyte is a substance that produces an electrically conductive solution when dissolved in water. Without electrolytes, batteries would not be able to function. The three primary functions of electrolytes in batteries are to provide a medium for electrical current, to store energy, and to help keep the battery’s internal environment stable.
| The most common type of electrolyte used in batteries is sulfuric acid | When sulfuric acid is mixed with water, it creates a solution that can conduct electricity. This solution is also capable of storing energy in the form of chemical bonds between the sulfuric acid molecules and the water molecules. |
| The third function of electrolytes is to help maintain a stable internal environment within the battery | This is accomplished by preventing the build-up of unwanted materials on the electrodes and by providing a barrier between the electrode surfaces and the electrolyte solution. |
How to Make Battery Electrolyte Solution?
Making your own battery electrolyte solution is a great way to save money and get the most out of your batteries. There are a few things you need to know before you start, but once you have the basics down it’s easy to make your own electrolyte solution at home. The first thing you need to know is what type of electrolyte solution you need for your batteries.
There are two main types of electrolytes, acidic and alkaline. Acidic solutions are typically used in lead acid batteries, while alkaline solutions are used in nickel-cadmium and nickel-metal hydride batteries. Each type of battery has its own specific requirements for the concentration of electrolyte solution, so be sure to check your owner’s manual or ask a professional before making your own solution.
Once you know what type of solution you need, gathering the supplies is easy. For an acidic solution, all you need is distilled water and sulfuric acid. For an alkaline solution, you’ll need potassium hydroxide or sodium hydroxide instead of sulfuric acid.
You can find these chemicals at most hardware stores or online retailers that sell battery supplies. Making the actual solution is simple: just mix together the distilled water and chemical in the appropriate ratio specified by your battery’s manufacturer. Once mixed, store the Solution in a cool, dark place until ready to use.
When it comes time to use your homemade Solution, simply pour it into your battery according to the manufacturer’s instructions. And that’s it! You’ve now saved yourself some money by making your own electrolyte Solution.
Lithium-Ion Battery Electrolyte
Lithium-Ion Battery Electrolyte
As the name suggests, lithium-ion batteries make use of lithium ions in order to function. These ions are found in a material known as an electrolyte, which is essential for the proper operation of the battery.
Without the electrolyte, the battery would be unable to store and release energy.
The electrolyte used in lithium-ion batteries is typically a liquid or gel. It consists of a mixture of solvents and salts that allow for the movement of ions between the electrodes of the battery.
The most common type of solvent used is ethylene carbonate, while common salt choices include lithium hexafluorophosphate or lithium perchlorate.
When choosing an electrolyte for a lithium-ion battery, it is important to consider its properties carefully. The properties that are most important include ionic conductivity, thermal stability, and compatibility with the materials used in the battery’s electrodes and casing.
A good electrolyte will allow for efficient energy storage and discharge while also being safe to handle and use over time.
Best Electrolyte for Battery
The best electrolyte for batteries is aqueous potassium hydroxide (KOH). This electrolyte has the highest electrical conductivity of any known electrolyte and is also non-corrosive. Potassium hydroxide is the preferred electrolyte for high-temperature applications such as automotive batteries.
Car Battery Electrolyte
A car battery is made up of a series of lead-acid cells connected together. The electrolyte in each cell is a sulfuric acid and water solution. The lead-acid cell has two plates, the positive plate (made up of lead dioxide) and the negative plate (made up of spongy lead).
When the car is running, the battery provides power to the starter motor and ignition system. It also supplies power to accessories like lights and the radio when the engine is off. When you start your car, your alternator charges the battery, replacing the power it lost while powering your car’s electrical systems.
Over time, sulfates build up on the plates and reduce the amount of surface area that can react with the electrolyte. This reduces the capacity of your battery and its ability to hold a charge. If you don’t regularly use your car or if it sits for long periods without being driven, this sulfation can become severe enough to render your battery unusable.
Where to Buy Battery Electrolyte?
If you’re looking for battery electrolytes, there are a few places you can buy them. Most hardware and home improvement stores sell battery acid, which is the most common type of battery electrolyte. You can also find it online at sites like Amazon.com.
When buying battery electrolytes, make sure to get the right kind for your needs. Different types of batteries require different types of electrolytes. Lead-acid batteries, for example, require sulfuric acid while nickel-based batteries require potassium hydroxide or sodium hydroxide.
Be careful when handling battery acid, as it can be dangerous if not used properly. Always wear gloves and eye protection when working with it, and be sure to follow the manufacturer’s instructions carefully.
Battery Electrolyte Autozone
When it comes to your car, there are a lot of things that need to be kept in good working order. One of the most important parts of your car is the battery. The battery is what provides power to your car and if it isn’t working properly, your car won’t run.
The electrolyte in your battery is what provides the power to your battery and if it isn’t working properly, your battery won’t work. That’s why it’s important to make sure that the electrolyte in your battery is always fresh and full.
One way to keep the electrolyte in your battery fresh and full is to use AutoZone’s Battery Electrolyte.
This product helps maintain the level of electrolyte in your battery so that it can continue to provide power to your car. It also helps extend the life of your battery by keeping it from overworking itself.
If you’re looking for a way to keep your car’s battery healthy and functioning properly, AutoZone’s Battery Electrolyte is a great option.
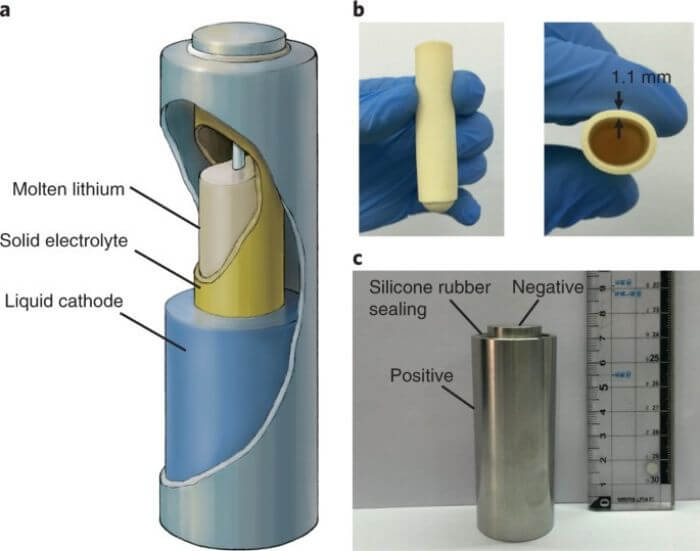
People Also Asked
What is the Use of Electrolytes in Batteries?
An electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water. The solute-free (pure) solvent is then called the “electrolyte”. An electrolyte in a battery is used to move electrons between the anode and cathode.
This movement of electrons creates an electric current. Batteries require a minimum amount of electrolytes to function. If there is not enough electrolyte, the battery will not work properly.
There are two types of electrolytes: liquid and gel. Liquid electrolytes are typically used in sealed lead acid batteries and NiCad batteries. Gel electrolytes are often used in Lithium-ion batteries.
What is the Function of Electrolytes in Lithium Ion Cells?
Lithium-ion batteries are used in a variety of electronic devices, including cell phones and laptops. The electrolyte in a lithium-ion battery is responsible for storing and releasing the lithium ions that power the device. Lithium ion batteries work by using the movement of these ions to create an electric current.
The electrolyte also helps to keep the lithium ions evenly distributed throughout the battery, which helps to ensure that the battery will work properly and last for a long time.
How Does an Electrolyte Work?
Electrolytes are a type of compound that conducts electricity when dissolved in water. They are made up of positive and negative ions that are attracted to each other. When an electrolyte is placed in water, the ions split apart and move to the oppositely charged electrodes.
This process is called electrolysis. Electrolytes are used in many different applications, including batteries, fuel cells, and electroplating. Batteries use electrolytes to create a chemical reaction that produces electricity.
Fuel cells use electrolytes to generate electrical power from chemical reactions between fuel and oxygen. Electroplating uses electrolytes to coat metals with a thin layer of another metal.
Final Verdict
An electrolyte is a liquid or gel that contains ions and can conduct electricity. In a battery, the electrolyte is the medium between the anode and cathode, where reactions take place that generates electrical energy. The purpose of an electrolyte in a battery is to facilitate these reactions by providing a path for electrons to flow between the electrodes.
The type of electrolyte used in a battery depends on the specific chemistry of the battery. For example, lead-acid batteries use sulfuric acid as their electrolyte, while lithium-ion batteries use a variety of organic solvents.