In 1887, German engineer Karl Benz invented the first internal combustion engine-powered car. Benz’s car had a low voltage electrical system that used an alkaline battery.
The first commercially successful alkaline batteries were developed in 1896 by French chemist Georges Leclanche.

The alkaline battery was invented in the early 1950s by a team of scientists working for the Eveready Battery Company. The first commercially available alkaline batteries were introduced in 1957. Since then, alkaline batteries have become the most popular type of disposable battery in the world.
Is Duracell Alkaline Battery?
Duracell’s alkaline batteries are some of the most popular on the market, and for good reason. They’re reliable, long-lasting, and affordable, making them a great choice for a wide variety of devices.
If you’re looking for an alkaline battery that will stand up to repeated use, Duracell is a great option.
Their batteries are designed to withstand being discharged and recharged multiple times, so you can rest assured that they’ll keep your devices powered up when you need them most.
What’s more, Duracell’s alkaline batteries are also reasonably priced, making them a great value for money. So if you’re looking for a dependable and affordable battery option, Duracell should be at the top of your list.
Alkaline Battery Vs Lithium
Alkaline batteries are the most common type of battery used in household items like flashlights and smoke detectors. Lithium batteries, on the other hand, are typically used in high-end electronics like cell phones and laptops. So which one is better?
Alkaline batteries have a longer shelf life and can withstand more extreme temperatures than lithium batteries. They’re also cheaper to produce. However, lithium batteries pack more power into a smaller package, making them ideal for use in portable devices.
If you’re looking for a battery to power your everyday household items, go with alkaline. But if you need a powerful battery for your electronic devices, lithium is the way to go.
What are Alkaline Batteries Used for?

Alkaline batteries are a type of primary battery that have an alkaline electrolyte. They are also known as button batteries when they are used in small, cylindrical shapes like those found in watches. The use of an alkaline electrolyte allows for a higher discharge rate and longer shelf life than other types of primary batteries.
Some common applications for alkaline batteries include: remote controls, smoke detectors, flashlights, digital cameras, and portable electronic devices. Alkaline batteries power many of the devices we use on a daily basis, so it’s important to know how to properly store and dispose of them. Here are some tips for storing and disposing of alkaline batteries:
-Store alkaline batteries at room temperature in a dry place. -Do not expose them to extreme heat or cold as this can shorten their lifespan. -When disposing of alkaline batteries, be sure to recycle them properly.
Do not throw them in the trash!
Are Alkaline Batteries Rechargeable?
Are Alkaline Batteries Rechargeable?
Yes, alkaline batteries are rechargeable! You can use a standard household charger to recharge them.
Just be sure to follow the manufacturer’s instructions, as overcharging can shorten the battery life.
There are also specialized alkaline battery chargers available on the market. These can be a good investment if you plan on recharging your batteries regularly, as they can help extend the life of the battery.
If you decide to recharge your alkaline batteries, just keep in mind that they won’t last as long as regular rechargeable batteries. So, it’s best to use them for occasional use rather than relying on them for daily tasks.
Alkaline Battery Disposal
Are you looking for information on how to dispose of alkaline batteries? If so, you’ve come to the right place. Alkaline batteries are a common type of battery that is used in many devices, including flashlights, remote controls, and toys.
Durable and Last Long
While they are durable and last long, eventually they will need to be replaced. When this time comes, it’s important to know how to properly dispose of them. There are a few options for disposing of alkaline batteries.
To Recycle Them
This can be done by taking them to a local recycling center or by mail-in recycling programs. Recycling helps reduce pollution and conserve resources.
To Landfill Them
This should only be done if the batteries are completely dead and can’t be recycled. Be sure to wrap the batteries in something like newspaper so they don’t short circuit and cause a fire hazard.
Reuse the Batteries if Possible
Many stores that sell alkaline batteries will take old ones back and recycle them for you. You can also check with your local city or county government about battery recycling programs in your area.
By reusing or recycling batteries, we can all do our part to help protect the environment!
Manganese Battery Vs Alkaline
There are many different types of batteries on the market today, each with its own advantages and disadvantages. Two of the most popular types are manganese batteries and alkaline batteries. So, which is the better option?
| Manganese batteries | Alkaline batteries |
| Manganese batteries are typically cheaper than alkaline batteries and have a longer shelf life. They also perform well in cold temperatures. However, manganese batteries can leak and damage devices if not used properly. | Alkaline batteries are more expensive than manganese batteries but have a shorter shelf life. They also don’t perform as well in cold temperatures. However, alkaline batteries are less likely to leak and damage devices. |
So, which battery is the best option? It really depends on your needs and budget. If you need a battery that is cheap and has a long shelf life, then a manganese battery may be the better option for you.
However, if you need a battery that is less likely to leak and damage your devices, then an alkaline battery may be the better choice.
Advantages of Alkaline Battery
Alkaline batteries have a number of advantages over other types of batteries. They are cheaper to produce, last longer and can be used in a wider range of temperatures. Alkaline batteries also have a higher energy density than other types of batteries, making them ideal for use in high-drain devices such as digital cameras.
Alkaline Battery Voltage
What is an alkaline battery? An alkaline battery is a type of primary battery which uses an alkaline electrolyte. The term “alkaline” refers to the basic nature of the solution, as opposed to acidic.
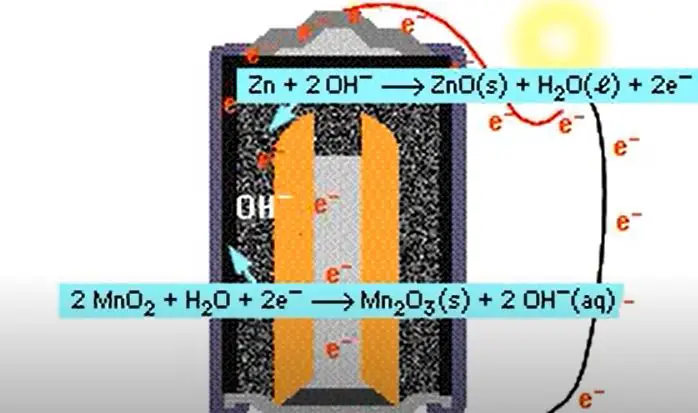
These batteries are sometimes also referred to as “Zinc-carbon batteries”. How do alkaline batteries work? Alkaline batteries work by using an electrolyte made up of potassium hydroxide and zinc oxide.
This electrolyte reacts with the zinc in the anode (negative electrode) to produce electrons. These electrons flow through the external circuit and into the cathode (positive electrode), where they react with oxygen to produce water. What are the benefits of using alkaline batteries?
Alkaline batteries have a number of advantages over other types of battery:
– They have a higher energy density, meaning they can store more energy per unit weight than other types of battery.
– They have a longer shelf life than other types of battery, meaning they will last for longer before needing to be replaced.
– They are less likely to leak than other types of battery, making them safer to use and transport.
– They can operate at a wider range of temperatures than other types of battery, meaning they can be used in both cold and hot environments.
How was the Alkaline Battery Invented?

In 1887, a French engineer named Georges Leclanché filed a patent for the first alkaline battery. His design used zinc and manganese dioxide as the electrodes, with an ammonium chloride solution as the electrolyte. This basic design is still in use today.
In 1896, German scientist Carl Gassner improved on Leclanché’s design by using a zinc-carbon electrode instead of zinc. This made batteries cheaper to produce and more powerful. Gassner’s batteries were widely used in flashlights and other portable devices.
In 1949, American chemist Lewis Urry developed a new type of alkaline battery that used calcium instead of manganese dioxide as the positive electrode. This made batteries even more powerful and longer lasting. Urry’s invention was crucial for the development of modern portable electronics such as laptops and cell phones.
Who Developed the Alkaline Battery?
The alkaline battery was invented by a man named Lewis Urry. He worked for the Eveready Battery Company, which is now known as Energizer. He developed the first alkaline battery in 1957.
Why Was the Invention of the Alkaline Battery Important?
The alkaline battery was invented in the 1950s by a team of researchers at the Eveready Battery Company. It was an important invention because it made batteries more efficient and longer lasting. Alkaline batteries are used in many devices today, including flashlights, radios, and portable electronic devices.
When Did Lewis Urry Invent the Alkaline Battery?
Inventor Lewis Urry is credited with developing the alkaline battery in 1959 while working for Eveready Battery Company (now known as Energizer). Alkaline batteries are a type of primary battery that offer longer shelf life and better performance than traditional zinc-carbon batteries. They are also more expensive to produce.
Uryu’s invention was inspired by his work on mercury oxide button cells, which were used in hearing aids and other small electronic devices. He realized that by using an alkaline electrolyte instead of a acidic one, he could create a battery with improved performance. The first commercial alkaline batteries were introduced in 1966.
Today, alkaline batteries are the most popular type of primary battery in the world, thanks to their reliability and reasonable cost. They are used in everything from flashlights and smoke detectors to remote controls and toys.
Conclusion
The alkaline battery was invented in the early 1950s by a team of scientists working for the British company, P.R. Mallory & Co. Ltd. The team was led by John Barden and also included Trevor Robinson and Alec Johnson.
The first alkaline batteries were made from zinc and manganese dioxide, which are still used in some types of batteries today.