There are many factors to consider when choosing an electrolyte for a battery. The electrolyte must be able to conduct electricity, and it must be stable at the operating voltage of the battery. It should also have a high boiling point to prevent evaporation, and it should be non-corrosive.

A good electrolyte for a battery is one that is able to conduct electricity and also has a high boiling point. The electrolyte must be able to hold onto the ions in the solution so that they are available to react with the electrodes in the battery.
What Makes a Good Solid Electrolyte?
In order for a solid electrolyte to be effective, it must display high ionic conductivity while also being mechanically and chemically stable. This means that the electrolyte should not corrode or degrade when in contact with other materials, and should be able to withstand repeated cycling (discharging and recharging) without performance degradation. The ideal solid electrolyte would also have a low density, making it lighter and therefore cheaper to produce.
How Do You Increase Electrolytes in a Battery?
In order to increase the electrolytes in a battery, you need to add more of the ions that make up the electrolyte solution. This can be done by adding more of the salt that is used to create the solution, or by adding other acids or bases to the solution. The concentration of the electrolyte solution can also be increased by evaporating some of the water out of the solution.
What is Used As an Electrolyte in a Battery?
An electrolyte is a substance that conducts electricity in an electrical circuit. It is usually a liquid or gel, but can also be a solid. In a battery, the electrolyte is the medium through which ions travel between the anode and cathode.
Ions are atoms that have gained or lost electrons, giving them a net charge. When two electrodes are placed in an electrolyte, they form what is known as an electrochemical cell. This cell produces electricity through a process called oxidation-reduction (or redox).
In a battery, the anode is made of metal that easily oxidizes (loses electrons), while the cathode is made of metal that easily reduces (gains electrons). As the cells discharge, the anode loses electrons and oxidizes, while the cathode gains electrons and reduces. The flow of ions through the electrolyte creates an electric current.
The most common electrolytes used in batteries are sulfuric acid, lead-acid batteries; potassium hydroxide, nickel-metal hydride batteries; and lithium-ion batteries.
How Do You Make an Electrolyte Solution?
An electrolyte is a solution that contains ions and can conduct electricity. Common electrolytes include sodium chloride ( NaCl), potassium chloride (KCl), and calcium chloride (CaCl2). To make an electrolyte solution, you will need to dissolve the desired amount of salt in water.
For example, to make a 1 molar (M) solution of NaCl, you would need to add 58.44 grams of NaCl to 1 liter of water.
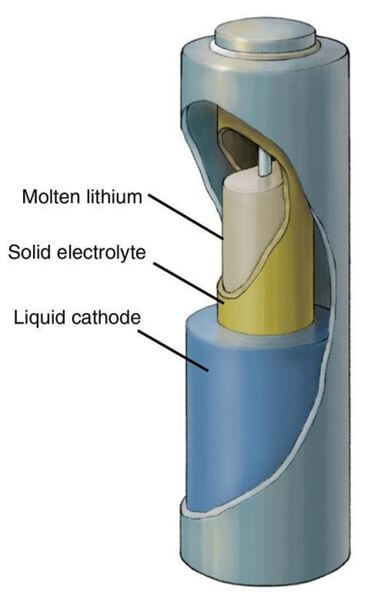
Best Electrolyte for Battery
When it comes to batteries, there are a lot of options on the market. But what is the best electrolyte for battery? The answer may surprise you.
First, let’s start with a little bit of background information. Batteries work by storing electrical energy and releasing electrons. When you plug in your phone or laptop, the battery is actually charging itself by taking in electrons from the outlet. Click here to know types of battery charging.
When you use your devices, the battery releases those same electrons to power your device. The key to a battery’s performance is the electrolyte. This is a substance that allows electrons to flow freely between the positive and negative electrodes.
Without an electrolyte, batteries would not be able to store or release electrons and would be useless. There are a variety of different electrolytes on the market, but not all of them are created equal. The best electrolyte for battery performance is lithium ion (Li-ion).
This type of electrolyte has several advantages over other types of electrolytes: 1) Li-ion batteries have a higher energy density and battery will last longer than other types of batteries. This means that they can store more energy per unit of volume than other types of batteries.
This makes them ideal for applications where space is limited, such as in portable electronic devices like cell phones and laptops. 2) Li-ion batteries have a higher power density than other types of batteries. This means that they can deliver more power per unit of volume than other types of batteries.
How to Make Battery Electrolyte?
Making your own battery electrolyte is a great way to save money and get the most out of your batteries. Here’s how to do it:
| 1. Gather your materials | You will need distilled water, lye (sodium hydroxide), and battery acid (sulfuric acid). You can find these materials at most hardware stores. |
| 2. Carefully measure out equal parts of water and battery acid | Pour these into a container that can be sealed tightly. |
| 3. Add lye to the mixture, a little at a time, until it stops fizzing | This means that the reaction between the lye and battery acid is complete and the resulting solution is now safe to handle. |
| 4. Once the reaction has stopped | Seal the container tightly and store it in a cool, dark place until you’re ready to use it. |
Lithium-Ion Battery Electrolyte
Lithium-ion batteries are common in today’s world. Many of our electronic devices, such as cell phones and laptops, rely on these batteries to function. But what exactly is a lithium-ion battery?
A lithium-ion battery is a type of rechargeable battery that contains lithium ions in its electrolyte. The electrolyte is a liquid or gel that contains the lithium ions and allows them to move between the anode and cathode of the battery during charging and discharging.
The chemical reaction that occurs during charging and discharging creates electricity, which powers our devices.
Lithium-ion batteries are popular because they are lightweight and have a high energy density, meaning they can store more charge than other types of batteries.
However, there are some drawbacks to lithium-ion batteries. They can be expensive, and they may pose a safety risk if not used correctly.
Improper charging or overloading can cause the battery to overheat, leading to fires or explosions.
Despite these risks, lithium-ion batteries are an important part of our modern world.
Use of Electrolyte in Battery
In a lead acid battery, the electrolyte is a mixture of water and sulfuric acid. This mixture allows for a chemical reaction to take place between the lead in the electrodes and the sulfuric acid, which creates an electric current.
The electrolyte also helps to keep the lead electrodes from corroding.
When the battery is not in use, the lead sulfate that is produced by the chemical reaction settles on the electrodes. If this sulfate was allowed to build up unchecked, it would eventually prevent the flow of electrons and render the battery useless.
The addition of water to the electrolyte keeps the concentration of sulfuric acid at a level where it can continue to react with the lead but does not allow for excessive corrosion of the electrodes.
The water also evaporates over time, which concentrates the sulfuric acid and increases its conductivity.
How to Make Lead Acid Battery Electrolyte Solution?
A lead acid battery is made up of cells that each contain a positive and negative plate separated by an electrolyte solution. The electrolyte solution is what allows for the flow of electrons between the plates and ultimately provides power for the battery. The most common electrolyte used in lead acid batteries is sulfuric acid, which can be corrosive and dangerous to handle.
However, with proper safety precautions, it is possible to make your own lead acid battery electrolyte solution at home. Here’s what you’ll need:
1. Distilled water;
2. Sulfuric acid (available at hardware stores);
3. A funnel;
4. A measuring cup or container;
5. Safety goggles and gloves;
1. Begin by adding distilled water to your measuring cup or container until it reaches the 1-liter mark.
2. Next, use the funnel to add sulphuric acid to the water until it reaches the 200ml mark – be very careful not to splash any of this on yourself as it can cause serious burns!
3. Once you’ve added all of the sulphuric acids, put on your safety goggles and gloves and give the mixture a good stir until it’s completely combined.
4. Your homemade lead acid battery electrolyte solution is now ready to use!
Battery Electrolyte for Sale
If you’re looking for a battery electrolyte for sale, there are a few things you need to know.
Step 1
First, what is a battery electrolyte? Battery electrolyte is a solution that conducts electricity and is used in lead-acid batteries.
It’s made up of water and sulfuric acid, and it’s what allows the battery to store and release energy.
Step 2
Second, why do you need it? If your lead-acid battery isn’t working properly, it might be because the electrolyte has dried out or become contaminated.
Replacing the electrolyte can help restore your battery to its original performance.
Step 3
Third, where can you buy it? You can find battery electrolytes for sale at most auto parts stores or online retailers that sell automotive supplies.
Be sure to check the product description to make sure you’re getting the right type of electrolyte for your particular battery.
Step 4
Finally, how do you use it? Once you’ve purchased the battery electrolyte, simply follow the instructions on the package.
In most cases, all you’ll need to do is add water to the solution and then pour it into your battery using the provided funnel. That’s all there is to it!
Battery Electrolyte Replacement
Your car’s battery is what provides power to the starter motor, which in turn starts the engine. The battery also powers all of the car’s electrical accessories when the engine is not running. A lead-acid battery contains six cells, each of which has a positive and negative plate separated by an electrolyte (sulphuric acid).
Over time, sulphuric acid breaks down and loses its ability to conduct electricity. This process is accelerated by high temperatures and vibration (from driving). When enough sulphuric acid has been lost, the battery will no longer be able to hold a charge and will need to be replaced.
The first step in replacing a battery is to disconnect the old one. You’ll need to remove the negative terminal first, followed by the positive terminal. Once the terminals are disconnected, you can lift out the old battery.
Before installing the new battery, it’s important to clean both terminal posts and also apply some grease or Vaseline to prevent corrosion. Once that’s done, you can connect up the new battery starting with the positive terminal first. Finally, tighten up both terminals before trying to start your car.
Battery Electrolyte Mixing Ratio
The Battery Electrolyte Mixing Ratio is a simple 1:1 ratio of water to battery acid. This mixing ratio will result in a working battery with an output of 12 volts. It is important to use distilled water when mixing the electrolyte, as impurities in tap water can damage the lead plates in the battery.
Once you have mixed the electrolyte, it is important to keep it tightly sealed in order to prevent evaporation. If you need to top up your battery acid levels, simply mix more water and battery acid together in the same 1:1 ratio and add it to the battery.
Wrapping Up a Conclusion
In order for a battery to work properly, it needs an electrolyte. This substance helps to conduct electricity between the positive and negative electrodes in the battery.
Some of the most common electrolytes used in batteries are potassium hydroxide, sodium hydroxide, and sulfuric acid. Potassium hydroxide is one of the most popular choices for battery electrolytes because it is highly conductive and relatively stable. However, it can be corrosive and may cause damage to the electrodes over time.
Sodium hydroxide is another common choice, but it is not as conductive as potassium hydroxide and can also be corrosive. Sulfuric acid is another option that is very conductive but also highly corrosive. For this reason, it is often diluted with water before being used in a battery.
Used Resources: