A battery is a device that stores energy and converts it into electrical current. The three main components of a battery are the anode, cathode, and electrolyte. The anode is the negative electrode, the cathode is the positive electrode, and the electrolyte is a conductive medium.
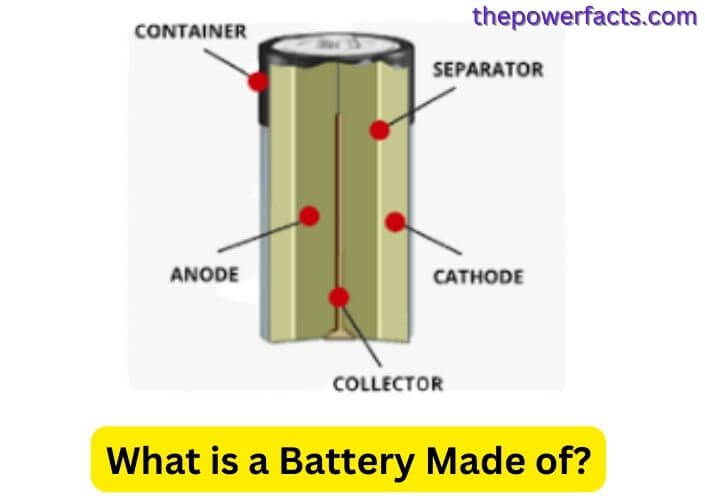
A battery is a device that converts chemical energy into electrical energy. The most common type of battery is the lead-acid battery, which consists of a series of connected cells. Each cell contains a positive and negative electrode separated by an electrolyte.
When the battery is in use, the electrodes react with the electrolyte to produce an electric current. The ingredients in a typical lead-acid battery are Lead (Pb), Cadmium (Cd), Mercury (Hg), Selenium (Se), Sulfuric acid (H2SO4), and Water (H2O) Each component plays an important role in the function of the battery.
Lead and cadmium are used for the electrodes because they have a high affinity for oxygen, which helps to prevent corrosion. Mercury is added to the electrolyte to help improve conductivity. Selenium is used as a catalyst to promote reactions between the electrodes and electrolytes.
Sulfuric acid is used as the electrolyte because it has a high ionic strength, which allows it to easily conduct electricity. Water is added to dilute the sulfuric acid and help keep it from freezing.
What is a Car Battery Made of?
A car battery is made up of cells that convert chemical energy into electrical energy. The most common type of cell used in car batteries is the lead-acid cell. Lead-acid cells are made up of a positive plate (made of lead dioxide) and a negative plate (made of pure lead).
These plates are separated by an electrolyte (a solution of sulfuric acid and water). When the engine is running, the alternator charges the battery, causing a reaction between the lead and lead dioxide on the positive plate. This creates electrons, which flow from the negative plate to the positive plate through an external circuit.
This flow of electrons provides power to start the engine and run accessories like lights and radios. The main advantage of lead-acid batteries is that they’re inexpensive to manufacture. However, they have some disadvantages as well.
For example, they’re relatively heavy, so they can add significant weight to a vehicle. They also tend to be less efficient than other types of batteries, so they need to be regularly recharged.
What is the Liquid Inside a Battery?
Batteries are made up of a number of different parts, all of which work together to create an electrical current. The most important part of a battery is the electrolyte, which is the liquid inside the battery that carries the electrical charge. Without the electrolyte, batteries would not be able to function.
The electrolyte is made up of a number of different chemicals, including sulfuric acid and lead oxide. These chemicals react with each other to create an electrical current that can power devices such as flashlights and computers. While the electrolyte is essential to the functioning of a battery, it is also dangerous.
If a person comes into contact with battery acid, it can cause serious burns. That’s why it’s important to be careful when handling batteries and to always keep them away from children and pets.
What are Rechargeable Batteries Made of?
Rechargeable batteries are made of a number of different materials, depending on the type of battery. The most common type of rechargeable battery is the lead-acid battery, which is made of lead and acid. But how many times can you charge a rechargeable battery before it needs to be replaced? The answer to this question depends on the type of battery and the quality of the charger.
Other types of rechargeable batteries include nickel-cadmium (NiCd) batteries, lithium-ion (Li-ion) batteries, and nickel-metal hydride (NiMH) batteries. Different battery sizes are needed for different devices.
How Does a Battery Work?
Batteries are a common power source for many devices, but how do they work? A battery is basically two electrodes (usually made of metal) submerged in an electrolyte solution. When the battery is connected to an external circuit, electrons flow from the negative electrode to the positive electrode through the electrolyte and the external circuit.
This flow of electrons creates a current that can be used to power devices. The chemical reaction that causes this flow of electrons is known as an oxidation-reduction reaction. The negatively charged electrode is called the cathode and the positively charged electrode is called the anode.
The cathode contains materials that are easily oxidized, while the anode contains materials that are easily reduced. When the battery is connected to a circuit, these reactions occur simultaneously: oxidation at the anode and reduction at the cathode. Oxidation occurs when electrons are lost from atoms at the anode.
This loss of electrons makes the atoms more positive, which makes them migrate towards the cathode. Reduction occurs when atoms at the cathode gain electrons, making them more negative. As these reactions occur, ions (atoms that have gained or lost electrons) move through the electrolyte solution between electrodes.
This movement of ions creates an electric current. The overall chemical reaction can be represented by this equation:
Anode: Reducing Agent → Oxidized products + Electrons
Cathode: Oxidizing Agent + Electrons → Reduced products
What are AA Batteries Made of?
Batteries come in all shapes and sizes, but they all have one thing in common: they need to be made of materials that can store and release electrical energy. So what are AA batteries made of? The answer depends on the type of battery.
There are two main types of AA batteries: alkaline and lithium. Alkaline batteries are the most common type of Aa battery. They’re made of zinc and manganese dioxide, with a small amount of potassium hydroxide added to the mix.
The zinc serves as the anode, or positive electrode, while the manganese dioxide is the cathode or negative electrode. When you use an alkaline battery (the first commercially successful alkaline batteries were invented in 1896 by French chemist Georges Leclanche), the chemical reaction between these materials produces electricity. Lithium AA batteries are less common than alkaline batteries, but they pack a lot more power into a smaller package.
That’s because they’re made with lithium metal instead of zinc. Lithium is a very reactive element, so it can store a lot more electrical energy than other metals like zinc. But this reactivity also makes lithium AA batteries more expensive than their alkaline counterparts.
So there you have it: Two different types of AA batteries made from different materials to achieve different results. No matter which type you choose, just make sure you recycle your old batteries when you’re done with them!
What are Tesla Batteries Made of?
Tesla batteries are made of lithium-ion cells. The cathode is usually made of nickel, cobalt, and manganese, while the anode is made of carbon. The electrolyte is a lithium salt in an organic solvent.
Commonly Used in Batteries
Batteries come in all shapes and sizes, but they all have one thing in common: they use a chemical reaction to create an electric current. This chemical reaction is usually between lead and acid, although other combinations are also possible. The first batteries were invented in the late 1700s by Italian physicist Alessandro Volta. The first car batteries were lead-acid batteries. They were invented in 1859 by French physicist Gaston Planté.
His invention, known as the voltaic pile, used a stack of metal disks separated by cloth soaked in brine (salt water). The top and bottom disks were made of different metals, such as copper and zinc. When Volta connected the two disks with a wire, he found that an electric current flowed through the wire.
Volta’s invention was improved upon in the 1830s by English chemist John Frederic Daniell. He replaced the brine-soaked cloth with a solution of sulfuric acid, which allowed for a more powerful battery. Daniell’s design was further improved upon by French physicist Georges Leclanche, who added a porous carbon rod to absorb the sulfuric acid solution. This became known as the Leclanche cell and is still used today in some applications.
Lead-acid batteries are perhaps the most well-known type of battery. They are often used in car engines to start them up (the technical term for this is “cranking”). Lead-acid batteries work by having two lead plates immersed in sulfuric acid; when these plates are connected together via a wire, an electric current flows and can be harnessed to do work such as cranking an engine.
What are Lithium Batteries Made of?
Lithium batteries are made of lithium, a metal with a low atomic number that is found in the Earth’s crust. Lithium has a high electrochemical potential, meaning it can easily give up electrons to create an electric current. This makes it ideal for use in batteries.
Lithium batteries are also very lightweight and have a high energy density, making them perfect for use in portable electronic devices.

Frequently Asked Question
What Chemicals are in a Battery?
Batteries are made up of a number of different chemicals, including lead, cadmium, nickel, and lithium. These chemicals work together to create an electrical current that can be used to power devices. Each type of battery has its own unique chemical makeup, which is what determines the strength and duration of the electrical charge it can provide.
What is the Main Material in a Battery?
Batteries are devices that store energy and convert it into a form that can be used to power electronic devices. The main material in a battery is the anode, which is made of metal oxide. The cathode is made of carbon.
The electrolyte is a solution of sulfuric acid and water.
Are Batteries Made of Lithium?
Lithium batteries are not only made of lithium but also of other materials like carbon and manganese. The positive electrode is made of lithium metal oxide, while the negative electrode is made of carbon. In between these two electrodes is an electrolyte solution, which helps to facilitate the flow of electrons between them.
Is Battery Made of Plastic?
No, batteries are not made of plastic. The material that makes up the battery’s casing is typically hard plastic, but the actual “battery” part is made of metal (usually lead) and acid.
Conclusion
Batteries are made up of a number of different materials, including metals like lead and copper, as well as chemicals like acid. The exact composition of a battery will vary depending on the type of battery it is, but all batteries have these basic components.