Batteries are one of the most important inventions in history. They are used to power everything from our cell phones to our cars. But what exactly is a battery and what does it do?
A battery is a device that stores energy and converts it into electricity. It consists of two or more electrochemical cells that produce an electric current when connected together. The first batteries were invented in the 1800s and were used to power things like telegraphs and light bulbs.
Today, batteries are used in a wide variety of devices, including laptops, smartphones, digital cameras, and even electric cars!
A battery is a device that stores energy and can be used to power devices. The three main functions of batteries are to store energy, convert chemical energy into electrical energy, and provide a power source for devices. Batteries come in many different shapes and sizes, and each type of battery has its own specific set of functions.
What are the Functions of a Battery?
A battery is a device that stores energy and releases it as electricity. Batteries come in many shapes and sizes, from the small button cell batteries used in hearing aids to the giant lead-acid batteries used in cars. All batteries have three basic parts: an anode (the negative end), a cathode (the positive end), and an electrolyte (a substance that helps conduct electricity).
When you connect a battery to an electrical circuit, electrons flow from the negative anode to the positive cathode through the electrolyte. This flow of electrons produces electricity. Batteries have two main functions: they store energy and release it as electricity.
Most batteries are made up of chemical reactions that produce electricity. The chemical reaction inside a battery can be either exothermic (releasing heat) or endothermic (absorbing heat). An exothermic reaction releases more energy than it takes to start the reaction, while an endothermic reaction requires more energy to get started than is released during the reaction.
They Store Energy
Batteries use both types of reactions to store and release energy. The function of a battery is determined by its chemistry. The most common type of battery chemistry is lead-acid, which is used in car batteries.
Release It as Electricity
Lead-acid batteries convert electrical energy in chemical form and then release it as electrical current when needed. Other types of battery chemistries include lithium ion, nickel metal hydride, and alkaline.
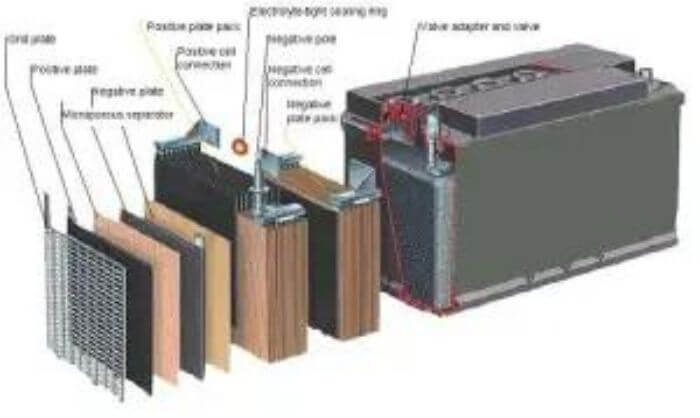
The Battery Consists of One Or More Cells That Store the Energy
Batteries are an essential part of many electronic devices, providing the power needed to operate them. A battery typically consists of one or more cells that store the energy, and a controller that regulates the flow of energy from the cell(s) to the device. The most common type of battery used in electronic devices is the lithium-ion (Li-ion) battery.
Li-ion batteries are lightweight and have a high energy density, meaning they can store a lot of energy in a small space. They also have a long lifespan and can be recharged many times before needing to be replaced. Other types of batteries used in electronic devices include lead-acid, nickel-cadmium (NiCd), and nickel-metal hydride (NiMH).
These batteries are generally heavier than Li-ion batteries and have lower energy densities. However, they may be more suitable for certain applications due to their different chemical properties. When choosing a battery for an electronic device, it is important to consider the specific needs of the device.
Factors such as size, weight, power requirements, and operating conditions will all affect which type of battery is best suited for the application.
Conclusion
Batteries are devices that store and release energy in the form of electricity. They are essential components of many electronic devices, including cell phones, laptops, and flashlights. Batteries have three primary functions: to store energy, convert chemical energy into electrical energy, and provide a power source for electronic devices.