The law of conservation of energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed, but it can be converted from one form to another. The kinetic energy of an object is its energy of motion, while the potential energy is the energy stored in the object due to its position or configuration.

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be conserved over time. This law is a direct consequence of the fact that the laws of physics are time-reversible. The total energy includes both kinetic energy, which is due to motion, and potential energy, which is due to position.
Is Law of Conservation of Energy Potential Or Kinetic?
The law of conservation of energy is the fundamental principle of physics that energy cannot be created or destroyed, only transformed from one form to another. This means that if you have a certain amount of potential energy, it can be converted into an equal amount of kinetic energy (or vice versa). The total amount of energy in the universe remains constant.
There are two types of energy –
| Potential energy | Potential energy is stored energy, like when you wind up a toy car before releasing it to watch it zoom across the floor. |
| Kinetic energy | Kinetic energy is movement energy, like when the toy car is actually zooming across the floor. |
The law of conservation of energy says that no matter what transformation occurs – whether it’s potential to kinetic or kinetic to potential – the total amount of energy in the system stays constant. In other words, if you start with 100 units of potential energy, after transforming some into kinetic energy, you’ll still have 100 units of combined energy in the system – just different forms of it.
What are the 2 Laws of Conservation of Energy?
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a fundamental principle of classical mechanics and it can be traced back to Gottfried Leibniz. It was also demonstrated by Joseph Lagrange in his formulations of mechanics.
The total energy of a system includes both its kinetic energy—the energy associated with its motion—and its potential energy—the stored energy due to the position of the objects within it. The law of conservation of energy states that, in an isolated system, these two energies will remain constant over time. In other words, the total amount of energy in a closed system always stays the same.
This principle has a number of important applications. For example, it helps us understand why it is difficult to create or destroy matter (as opposed to simply changing its form). It also explains why we cannot create perpetual motion machines: if such a machine could exist, it would violate the law of conservation of energy and thus could not continue to operate indefinitely.
What is a Conserved Sum of Potential And Kinetic Energy?
In classical mechanics, the total energy of a system is conserved. This means that the sum of the potential and kinetic energies in a closed system remains constant. Potential energy is the energy stored in a system due to its position or configuration.
Kinetic energy is the energy associated with the motion of objects within a system. In an isolated system, such as the universe, the total energy is said to be conserved because there are no external forces acting on it.
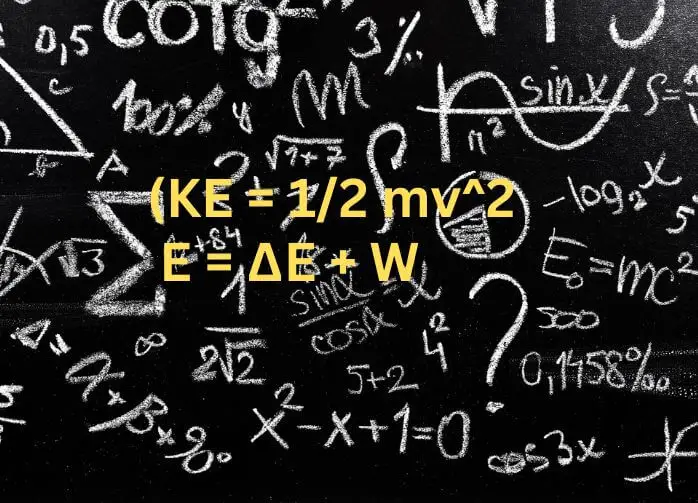
What is Kinetic Energy Conservation?
In physics, the conservation of kinetic energy states that the total mechanical energy of a closed system remains constant. This principle applies to a wide variety of situations, including collisions, where kinetic energy is converted into other forms of energy such as heat or sound. The law of conservation of energy is one of the most fundamental laws in all of physics.
The concept of kinetic energy conservation comes from the fact that momentum is conserved in all directions in an isolated system (one not subject to external forces). In classical mechanics, momentum is defined as the mass times velocity. Since velocity is a vector quantity (it has both magnitude and direction), momentum is also a vector quantity.
The law of conservation of momentum states that the total momentum of a closed system remains constant. If there are no external forces acting on a system, then the only way for the total momentum to change is if there is interaction within the system itself. For example, when two billiard balls collide, they exchange momentum with each other; as a result, their total momentum before and after the collision must be equal.
However, since kinetic energy depends on velocity squared (KE = 1/2 mv^2), it follows that if two objects collide and exchange kinetic energy with each other, their total kinetic energies before and after the collision will also be equal. Therefore, we can say that in an isolated system with no external forces acting on it, Kinetic Energy is Conserved.
Law of Conservation of Energy
The law of conservation of energy is a physical law that states that the total amount of energy in an isolated system remains constant. The total amount of energy can be converted from one form to another, but it cannot be created or destroyed. This law is also sometimes called the first law of thermodynamics.
Thermodynamics is the study of heat and energy. The laws of thermodynamics tell us how energy behaves in systems. The law of conservation of energy is a fundamental law of physics.
It has been tested many times and found to be true. Conservation laws like this one are important because they help us understand how systems work. In 1847, James Prescott Joule did experiments that showed that when work is done on a system, heat is always produced.
From these experiments, Joule concluded that there was a relationship between heat and mechanical work. He found that a certain amount of mechanical work always produces the same amount of heat. Joule’s experiments led to the development of the science of thermodynamics.
In 1851, William Rankine published the first book on thermodynamics. Rankine’s book included the concept of “potential energy.” Potential energy is stored energy that can be used to do work.
State the Law of Conservation of Energy With Example
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a fundamental principle of classical physics. The total energy includes kinetic energy, which is the motion energy of the particles; and potential energy, which is stored energy due to the configuration of the system (gravitational, electrical, etc).
Energy cannot be created or destroyed; it can only change form. For example, consider a ball rolling down a hill. As it rolls, its kinetic energy increases as its speed increases (KE = 1/2 mv^2).
At the same time, its gravitational potential energy decreases as it gets closer to the bottom of the hill (PE = mgh). But since these two types of energies are connected—they are both forms of mechanical energy—the total amount of mechanical energy stays constant.
What are the 3 Laws of Conservation of Energy?
The three laws of conservation of energy are as follows:
| Law 1 | Energy can neither be created nor destroyed; it can only be transformed from one form to another. |
| Law 2 | The total amount of energy in the universe is constant. |
| Law 3 | Energy cannot be created or destroyed in an isolated system; it can only be transferred from one object to another or converted from one form to another. |
Law of Conservation of Energy Formula
The Law of Conservation of Energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed, but only transformed from one form to another. The law is a consequence of the fact that energy is a conserved quantity; that is, it cannot be created or destroyed, but only changed from one form to another.
The law of conservation of energy can be stated as follows: In an isolated system, the total amount of energy remains constant over time. The law applies to all forms of energy, including thermal energy, kinetic energy, potential energy, and electrical energy. The law of conservation of energy is also known as the first law of thermodynamics.
It is a fundamental principle of classical mechanics and cannot be derived from any other laws. Thelawofconservationofenergyformulacanbeexpressedas follows E = ∆E + W where E is the total amount of energy in the system,∆Eis the change in energy of the system over time, and is the work done by the system on its surroundings over time.
Potential Energy And Conservation of Energy
When we think about energy, we often think of it as something that is always in motion. However, energy can also be stored. This stored energy is called potential energy.
Potential energy can be kinetic (energy of motion) or chemical (energy stored in atoms).
The law of conservation of energy states that energy cannot be created or destroyed, but only transformed from one form to another. This means that the total amount of potential and kinetic energy in the universe is always constant.
The most important aspect of potential energy is that it has the ability to do work. Work is done when a force moves an object through a distance. The more potential energy an object has, the more work it can do.
For example, a boulder at the top of a hill has more potential energy than a boulder at the bottom because it can do more work if it were to roll down the hill. The same goes for water behind a dam – it has lots of potential energy because it can do work by powering turbines and generating electricity.
State the Law of Conservation of Energy Class 9
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a fundamental principle of physics. It states that energy can neither be created nor destroyed; rather, it can only be transformed from one form to another.
The total amount of energy in the universe is thus always constant. The law of conservation of energy is often stated as E = mc2, where E is energy, m is mass, and c is the speed of light in a vacuum. This equation was first derived by Albert Einstein in his theory of special relativity.
In general relativity, the law takes on a more general form, which states that the total amount of energy in any closed system (including gravitational potential energy) remains constant. The law of conservation of energy has a number of important implications.
| 1st implications | First, it implies that Energy can neither be created nor destroyed. |
| 2nd implications | Second, it implies that The total amount of Energy in the universe is fixed. |
| 3rd implications | Third, it implies that Energy can only be converted from one form to another. |
| 4th implications | fourth, because the Law Of Conservation Of Energy says matter and radiation (light) cannot be created or destroyed, then we know there was no Big Bang and hence no Universe! |
Describe the Law of Conservation of Mechanical Energy

The law of conservation of mechanical energy states that the total mechanical energy in a closed system remains constant. Mechanical energy is the sum of kinetic and potential energies. This law applies to both conservative and non-conservative forces.
In a closed system, there are no external forces acting on the system. The only force acting on the system is gravity, which is a conservative force. The law of conservation of mechanical energy states that the total mechanical energy in a closed system remains constant.
The total mechanical energy in a system can be divided into two types:
Kinetic Energy: Kinetic energy is the motion energy associated with objects in motion.
Potential Energy: Potential energy is stored energy due to an object’s position or configuration.
Conclusion
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law means that energy can neither be created nor destroyed; it can only be transformed from one form to another. The total amount of energy in a system can change, but only because it has been transferred to or from another system.
The law of conservation of energy is also known as the first law of thermodynamics. Thermodynamics is the study of heat and its relationship to other forms of energy, such as work and mechanical energy. The first law simply states that heat and work are two forms of energy transfer: when heat flows into or out of a system, its internal Energy changes by an equal amount.
If no heat flows into or out of a system (that is, if it is isolated), then its internal Energy does not change—it remains constant over time. There are two types of Energy: kinetic and potential. Kinetic Energy is the Energy associated with motion; it’s the Energy you have when you’re moving.
Potential Energy is stored Energy; it’s the Energy you have when you’re not moving yet but have the potential to move (like when you wind up a toy car before releasing it).
Relevant Resources: