Published on: September 16, 2022
Written by Jonas Frank / Fact-checked by Nova Scarlett
The law of conservation of energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed, but only converted from one form to another. This means that the total amount of energy in the universe is always constant.
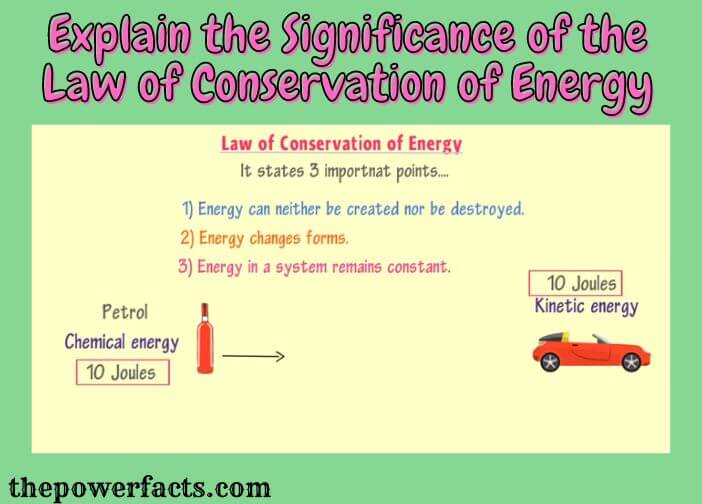
The law of conservation of energy is a fundamental principle of physics and it has many applications in our everyday lives.
The Law of Conservation of Energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed, but only converted from one form to another. This means that the total amount of energy in the universe is always constant.
This law has several important implications.
| First | it means that energy can never be created or destroyed. This is a very powerful idea because it means that the universe is fundamentally stable. |
| Second | it means that we can never run out of energy. Even though individual forms of energy may decline, the overall supply is always constant. |
| Finally | most importantly, it tells us that energy cannot be created or destroyed without violating the laws of physics. |
In other words, if someone claimed to have invented the machine that could create energy out of nothing, we would know immediately that they were wrong! The Law of Conservation of Energy is one of the most important principles in physics.
State the Law of Conservation of Energy
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a cornerstone of the sciences and it can be applied to a wide variety of situations. In simple terms, the law states that energy can neither be created nor destroyed; it can only change form.
The law of conservation of energy is often used in conjunction with the first law of thermodynamics. The first law of thermodynamics states that energy cannot be created or destroyed, but it can be transferred from one place to another or converted into different forms. When these two laws are combined, they state that the total amount of energy in the universe is always constant.
The law of conservation of energy has some important implications.
First: It means that energy can never be created or destroyed; it can only exist in different forms and be transferred between systems.
Second: It means that the universe is a closed system; no new matter or energy can enter or leave the system (except for gravitational effects).
Third: It implies that there must be a balance between kinetic and potential energy in any closed system; if one type of energy becomes too large relative to the other, then eventually there will be a transfer of energy to equalize them (this is how equilibrium is reached).
There are many real-world examples of the law of conservation of energy. One example is when a ball is dropped from a height; the potential energy of the ballet resting at the top of the drop is converted to kinetic energy as the ball falls and eventually reaches equilibrium at ground level where all its kinetic energy has been dissipated as heat.
Another example is photosynthesis in plants, which converts light into chemical potential energy that can later be used by animals through respiration.
State the Law of Conservation of Energy Class 9

The law of conservation of energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed. The total amount of energy in the universe is always constant.
This law has many important implications. For example, it means that we can never create unlimited energy. We can only convert energy from one form to another.
Heat is a type of energy that can be converted into other forms of energy, such as electricity or mechanical work. However, once the heat is converted into another form of energy, it cannot be converted back into heat. This is why it is not possible to create perpetual motion machines – because they would violate the law of conservation of energy!
State the Law of Conservation of Energy With Example
The law of conservation of energy is one of the basic laws of physics and states that energy can neither be created nor destroyed. The total amount of energy in a system remains constant, although it may be transformed from one form to another. For example, a ball rolling down a hill loses gravitational potential energy but gains kinetic energy.
In an isolated system, the law of conservation of energy guarantees that the total amount of energy stays constant. However, in an open system like our universe, there can be exchanges of energy with the surroundings. The most important example is the radiation of heat into space by stars like our Sun: as they convert nuclear fuel into heat and light, they also gradually lose some of their mass through this process.
Describe the Law of Conservation of Mechanical Energy

In physics, the law of conservation of mechanical energy states that the total mechanical energy of an isolated system remains constant—it is said to be conserved over time. This law derives from the first law of thermodynamics, which states that energy can neither be created nor destroyed; rather, it can only be transformed from one form to another. In addition to its intrinsic value in describing physical systems, the law of conservation of mechanical energy often proves to be a useful tool for solving problems in classical mechanics.
The total mechanical energy of a system includes both its kinetic energy—the energy associated with its motion—and its potential energy—the stored energy due to the position of its various parts relative to each other or relative to some external reference point. If there are no forces acting on the system other than conservative forces (such as gravity), then the total mechanical energy will remain constant over time. This means that if we know the total mechanical energy at any given instant, we can predict what it will be at any future or past time simply by calculating the change in kinetic and potential energies over that interval.
What are the 3 Laws of Conservation of Energy
In physics, the 3 law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a consequence of the fact that energy can neither be created nor destroyed; rather, it can only be transformed from one form to another. The total amount of energy in a system can thus change only by virtue of its interaction with other systems.
For example, if heat flows into or out of a closed system, then the internal energy of that system will change accordingly; however, since there are no other interactions taking place, the total amount of energy in both the system and its surroundings must remain constant. The law of conservation of energy is also known as the first law of thermodynamics. It was formulated by German physicist Hermann von Helmholtz in 1847 and later generalized by James Clerk Maxwell in 1871.
In addition to being applicable to closed systems, this law also applies to open systems that exchange both matter and energy with their surroundings.
If Energy Cannot Be Created Or Destroyed, Where Did It Come from?
In the most basic sense, energy is the ability to do work. It exists in several forms, including thermal (heat), electrical, chemical, nuclear, and mechanical. Energy can be converted from one form to another, but it cannot be created or destroyed.
The law of conservation of energy states that the total amount of energy in an isolated system remains constant—it is neither created nor destroyed. Where did all this energy come from? In a word: the Big Bang.
According to our best scientific understanding, approximately 14 billion years ago, all matter in the universe was concentrated in a very small space with incredibly high temperatures and densities. Then, for reasons not fully understood by scientists (but which may have something to do with quantum fluctuations), that space suddenly expanded rapidly—the Big Bang. As it did so, it cooled and matter began to form.
The expansion of the universe continues today and appears to be accelerating. As space expands, so does the amount of available energy because there is more volume in which photons (light particles) can travel. This means that there is more potential for interaction between particles—and thus more opportunity for work to be done.
Law of Conservation of Energy
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a consequence of the fact that energy can neither be created nor destroyed; rather, it can only be transformed from one form to another. For example, in a closed system chemical reaction, the total energy of the reactants must equal the total energy of the products.
The first person to formulate this law was Julius Robert von Mayer, a German physician, and physicist, who published his work in 1842. However, it was James Joule who later gave this law its name when he discovered that mechanical work could be converted into heat. In addition to these two men’s contributions, many other scientists have helped to develop and refine our understanding of energy conservation.
One important real-world application of the law of conservation of energy is in the field of thermodynamics. Thermodynamicists use this law to study how heat flows between different objects and how much work can be extracted from heat sources like steam engines.
Who Discovered the Law of Conservation of Energy?
In 1845, German physician Julius Robert von Mayer discovered the law of conservation of energy while investigating human metabolism. The law states that energy can neither be created nor destroyed, but it can be converted from one form to another. This discovery was an important step in the development of the modern understanding of thermodynamics.

People Also Asked
What is the Significance of the Law of Conservation of Energy?
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can neither be created nor destroyed; rather, it transforms from one form to another. The law of conservation of energy is a fundamental concept in both classical and modern physics; its applications extend far beyond mechanics.
The law of conservation of energy can be traced back to the early 19th century when French physicist Nicolas Léonard Sadi Carnot first proposed that heat was a form of motion and thus could not be created or destroyed. This idea led German physicist Hermann von Helmholtz to formulate the law in its current form in 1847. The law of conservation of energy has since been found to apply to a wide variety of physical and chemical systems, including mechanical systems, electrical circuits, nuclear reactions, and even biological processes.
In each case, the total amount of energy remains constant. The significance of the law lies in its ability to explain a wide range of physical phenomena. For example, the fact that moving objects tend to slow down over time can be explained by the fact that they are losing energy as they move—this loss is due to friction between the object and its surroundings.
Similarly, when two objects collide, their total kinetic energy remains constant; however, some of this kinetic energy is converted into other forms such as heat or sound. The law also has important implications for our understanding of how stars shine and how we can harness solar power here on Earth. Stars produce light and other electromagnetic radiation through nuclear fusion reactions, which convert hydrogen atoms into helium atoms.
These reactions release vast amounts of fusion power—the Sun emits around 386 billion trillion watts (3×1026 W)of power every second! However, according to the law of conservation of energy, this power must come from somewhere—in this case, it comes from the gravitational potential energy of star material as it falls inward under gravity toward the star’s core. Once again we see how different forms of the same underlying quantity (energy) can be converted into each other.
What is the Significance of the Law of Conservation of Energy Quizlet?
In physics, the law of conservation of energy states that the total amount of energy in an isolated system remains constant—it is said to be conserved over time. This law is a consequence of the fact that energy can neither be created nor destroyed; it can only be transformed from one form to another. The total amount of energy in the universe is thus always constant.
The law of conservation of energy is one of the most important laws in physics. It helps us to understand the behavior of systems and predict how they will change over time. For example, when a ball is thrown into the air, we know that it will eventually come back down because the gravitational potential energy is converted into kinetic energy as it falls.
Similarly, when a roller coaster reaches the top of a hill, we know that it will start rolling downhill because its gravitational potential energy is being converted into kinetic energy. The law of conservation of energy also has important implications for our understanding of thermodynamics, which is the study of heat and its relationship to work and other forms of energy. The first law of thermodynamics states that heat and work are both forms of Energy, but they cannot be interconverted—work can only be done if there is a temperature difference between two objects (for example, when you rub your hands together to generate heat).
The second law of thermodynamics states that entropy (a measure of disorder) always increases over time in an isolated system—this means that Energy always becomes less available for work as time goes on.
Conclusion
The law of conservation of energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed. This means that the total amount of energy in the universe is always constant.
The law of conservation of energy is a fundamental law of physics and it has many applications in our everyday lives.