A battery is made up of one or more cells that convert chemical energy into electrical energy. Each cell has at least two electrodes, a positive and a negative electrode, also known as the anode and cathode. The anode is where oxidation occurs and the electrons flow out of the cell to do work.
The cathode is where reduction occurs and electrons flow into the cell. In between the anode and cathode is an electrolyte material that allows ions to flow between them.
We all know how batteries work. We put them in our devices and they power them up. But how do the battery plates actually work?
The battery plate is made up of two metal plates, one positive and one negative. These are separated by a thin layer of material called an electrolyte. When we put a battery in our device, the two metal plates come into contact with each other and create an electrical current.
This current flows through our device and powers it up!
But what happens when the battery runs out of juice? The answer lies in the chemical reaction that takes place between the two metal plates and the electrolyte.
When the battery is running low, this reaction starts to slow down and eventually stops altogether. This is why our devices gradually lose power as the battery gets low – because the reaction that produces the electrical current is no longer taking place at full speed.
So there you have it – that’s how battery plates work!
How to Calculate Battery Plates?
Assuming you would like a blog post discussing how to calculate the number of battery plates needed dimensions of your battery and given for an application: Batteries are made up of one or more cells, each of which is composed of positive and negative electrodes (aka, battery plates) separated by an electrolyte. In order to determine how many battery plates are required for a given application, you must first understand the basics of electricity and electrochemistry.
Electricity is the flow of electrons through a conductor. The rate at which electrons flow is called amperage or current. The force that drives electrons through a conductor is called voltage.
Voltage can be thought of as the pressure pushing electrons through a circuit. The unit for measuring voltage is the volt (V). Electrochemistry is the study of chemical processes that occur at electrode/electrolyte interfaces.
Batteries rely on electrochemical reactions to generate an electric current. These reactions involve the transfer of electrons between two substances in contact with each other, typically metals and their ions in solution (aka, electrolytes). The number of battery plates required for a given application depends on the desired amperage and voltage output as well as the chemistry of the electrodes and electrolytes being used.
For example, lead-acid batteries typically use lead dioxide (PbO2) as the positive electrode and spongy lead (Pb) as the negative electrode. These electrodes are submerged in an electrolyte solution made up of water and sulfuric acid (H2SO4). In general, increasing either the surface area or active material loading on both electrodes will result in increased power output from the battery.
However, there are practical limits to how much surface area or active material can be used without compromising other aspects of battery performance such as life cycle efficiency or durability. Therefore, optimizing electrode design to achieve desired power output while minimizing cost and weight is critical for the successful commercialization of any new battery technology.
Car Battery Plates
If your car battery is more than three years old, it’s time to check the condition of the battery plates. The plates are the positive and negative electrodes that provide the electrical current for starting your engine and powering your accessories. Over time, these plates can become corroded, which reduces their ability to produce a strong electrical current.
If you notice any corrosion on the battery plates, it’s important to clean them as soon as possible. You can do this yourself with a simple household cleaning solution of baking soda and water. Just make sure you disconnect the battery before starting any work. Because disconnecting your battery will save power.
Once you’ve cleaned off the corrosion, it’s a good idea to apply a thin layer of petroleum jelly to the plates. This will help protect them from future corrosion and keep them working properly for years to come.
Automotive Battery Parts And Functions
An automotive battery is a type of rechargeable battery that supplies electrical energy to a vehicle. It is also known as a lead-acid battery. A car battery generally has a lifespan of about four to five years.
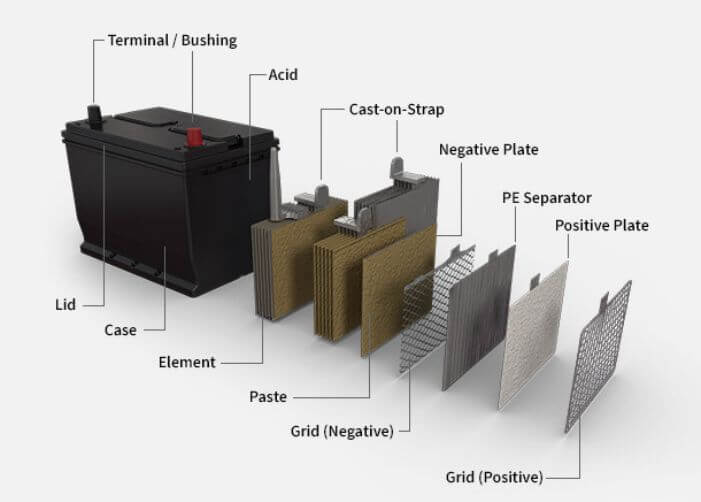
The main parts of an automotive battery are the positive and negative electrodes, separator, electrolyte, and terminal posts. The positive electrode is made of lead dioxide, while the negative electrode is made of spongy lead. The separator is usually made of fibrous material that prevents the two electrodes from coming into contact with each other.
The electrolyte is a mixture of water and sulfuric acid, which allows ions to flow between the electrodes when the battery is in use. The terminal posts are made of lead or brass and provide a connection point for the electrical circuit. When an automotive battery is not in use, it discharges itself slowly over time due to a process called self-discharge.
Self-discharge happens when the electrolyte reacts with the materials in the electrodes, causing them to break down and release electrons. This process can happen even when the vehicle’s engine is turned off and no external load is placed on the battery. To prolong the life of your automotive battery, it’s important to keep it charged.
You can do this by regularly starting up your vehicle and driving it for at least 30 minutes at a time.
What Type of Battery is a Car Battery?
A car battery is a lead-acid battery. It consists of a series of lead plates immersed in an acidic solution. When the engine is running, the alternator charges the battery, which provides power to the starter motor and other electrical accessories.
When the engine is not running, the battery provides power to these same accessories. The typical car battery has six cells, each of which produces 2 volts for a total of 12 volts. The capacity of a car battery is measured in amp-hours (Ah).
Most car batteries have a capacity between 45 and 60 Ah. Lead-acid batteries are classified as deep cycle batteries. This means that they can be discharged and recharged many times without damaging them.
In fact, it is good for a lead-acid battery to be regularly discharged and recharged as this helps to keep it functioning at its best.
How Do Car Batteries Charge?
Most car batteries (the average car battery lasts between two and five years) are lead-acid batteries, which use a chemical reaction between lead and sulfuric acid to create an electrical charge. During discharge, the lead sulfate crystals that are created can prevent the battery from being recharged. The charging process breaks down these lead sulfate crystals and reforms them into their original lead and sulfuric acid molecules.
This restores the electrical charge in the battery so it can be used again. There are two main types of chargers for car batteries: trickle chargers and standard chargers. Trickle chargers provide a slow, constant flow of electricity to the battery, which is ideal for long-term storage.
Standard chargers provide a higher level of electricity, which is necessary for recharging a battery that has been completely discharged. It’s important to choose the right charger for your car battery because using the wrong type of charger can damage the battery or even cause it to explode. If you’re not sure which type of charger to use, consult your car’s owner manual or ask a qualified mechanic.
What are Plates in Batteries?
Batteries are a type of electrical device that converts chemical energy into electricity. The three primary types of batteries are lead-acid, nickel-cadmium (NiCd), and lithium-ion (Li-ion). 6-cell Li-Ion batteries can actually last quite a long time if they are properly cared for. Each battery has its own unique set of characteristics, but they all have one thing in common: they rely on a series of positive and negative electrodes (known as “plates”) to create an electric current.
The positive plate is typically made of lead dioxide, while the negative plate is usually made of graphite. These plates are separated by an electrolyte (usually sulfuric acid) and are connected to the terminal posts of the battery. When the battery is discharged, electrons flow from the negative plate to the positive plate through the electrolyte.
How Does a Battery Work?
How Does a Battery Work? Batteries are devices that store and release electrical energy. The most common type of battery is the dry cell, which uses a variety of materials to create an electrochemical reaction. In 1800, Alessandro Volta invented the battery. The battery was invented in Como, Italy.
This reaction produces electrons, which flow through a circuit and power whatever device the battery is connected to. The three main components of a battery are the anode (the negative pole), the cathode (the positive pole), and the electrolyte (a substance that conducts electricity). The anode is made of carbon, while the cathode is usually made of metal oxide.
The electrolyte is typically a mixture of sulfuric acid and water. When the battery is not in use, electrons build up on the anode. When the battery is turned on, these electrons flow through the circuit to power whatever device it’s connected to.
As they flow, they combine with ions in the electrolyte and create a chemical reaction that produces heat and light. This reaction also causes the anode to gradually break down, which reduces its efficiency over time.
FAQs
What Do the Plates Become When the Battery is Discharged?
When a battery is discharged, the plates become covered in lead sulfate. Lead sulfate is an insoluble compound that forms when lead reacts with sulfuric acid. This substance can build up on the plates and prevent the battery from being able to hold a charge.
If left unchecked, this process will eventually destroy the battery.
What Happens to Plates When a Lead Acid Battery is Charged?
When a lead acid battery is charging, the positive and negative electrodes are reversed. The lead dioxide on the positive electrode (the anode) is converted into a metallic lead, while the lead on the negative electrode (the cathode) is converted into lead sulfate. These reactions result in a transfer of electrons from the anode to the cathode, which creates an electric current.
What are the Two Types of Battery Plates?
There are two types of battery plates: positive and negative. The positive plate is usually made of lead, while the negative plate is usually made of lead dioxide. The positive plate has a higher voltage than the negative plate, so when the two are connected, electrons flow from the positive to the negative plate.
This creates an electric current that can be used to power electrical devices.
What is the Function of Plates in Lead Acid Battery?
The function of lead acid battery plates is to provide a surface for the exchange of electrons between lead and acid. The lead oxide layer on the positive plate provides a site for the reduction of oxygen from the electrolyte, while the metallic lead on the negative plate serves as a site for the oxidation of hydrogen ions.
How are the Plates in a Lead Acid Battery Arranged?
There are two types of lead acid batteries, VRLA (valve regulated lead acid) and flooded. In a VRLA battery, the plates are arranged in a tightly packed stack with separators between them. The separators allow for ionic flow between the positive and negative plates while preventing physical contact between them.
This type of battery is sealed and maintenance-free. Flooded lead acid batteries have the same type of plate arrangement, but they are not sealed. These batteries require regular maintenance, such as adding water to the cells to keep the plates submerged.
Conclusion
Batteries are devices that store energy and convert it into electricity. The three main parts of a battery are the anode, cathode, and electrolyte. The anode is the negatively charged plate, the cathode is the positively charged plate, and the electrolyte is a solution that conducts ions between the two plates.
When a battery is connected to an electrical circuit, electrons flow from the negative plate to the positive plate through the electrolyte. This flow of electrons generates an electric current. The amount of electric current produced by a battery depends on the number of electrons flowing through the electrolyte per unit of time.
References: