Lithium batteries have become increasingly popular in recent years due to their high energy density and long life span. Lithium is a key ingredient in these batteries, and it can be found in a variety of minerals. The most common mineral used in lithium batteries is spodumene, which is mined in Australia, Brazil, and China.
Other minerals that are sometimes used include lepidolite, petalite, and amblygonite.

Lithium batteries are a type of rechargeable battery that uses lithium metal as an anode. Lithium batteries are commonly used in portable electronic devices, such as laptops, cell phones, and digital cameras. The cathode of a lithium battery is typically made from a transition metal oxide, such as cobalt oxide or manganese dioxide.
The anode is usually made from carbon, but can also be made from lithium metal. The electrolyte in a lithium battery is usually a mixture of organic solvents, such as ethylene carbonate or diethyl carbonate. Lithium salts, such as LiPF6 or LiClO4, are added to the electrolyte to help conduct electricity between the electrodes.
Lithium batteries have a high energy density and are lightweight, making them ideal for use in portable electronic devices. However, they can be expensive to produce and may pose environmental risks if not disposed of properly.
Is Lithium a Mineral?
Lithium is a mineral that has been used for centuries to treat a variety of mental health conditions. More recently, it has become a popular treatment for bipolar disorder. Lithium is thought to work by stabilizing mood swings and reducing the frequency and severity of manic episodes.
It is also used to treat depression and anxiety.
Lithium occurs naturally in some rocks and minerals, and can also be found in small amounts in certain hot springs. It is not considered an essential nutrient for humans, but lithium supplements are sometimes used to treat medical conditions.
Lithium side effects can include nausea, vomiting, diarrhea, tremor, headache, and dizziness.
Mining Minerals for Batteries
Batteries are an essential part of our lives. They power our phones, laptops, and even some cars. But have you ever wondered where the minerals in batteries come from?
Mining is the process of extracting minerals from the earth. And while there are many different types of mines, they all have one thing in common: they require a lot of energy to operate. That’s where batteries come in.
Batteries are used to power the equipment that mines use to extract minerals from the earth. And while mining can be a dirty and dangerous business, it’s necessary for us to get the minerals we need for our modern lives. There are many different types of minerals used in batteries, but some of the most important ones are lithium, cobalt, and nickel.
Lithium is used in rechargeable batteries like those found in phones and laptops. Cobalt is used in cell phone batteries and Nickel is used in rechargeable AA and AAA batteries. Mining these minerals can be difficult and dangerous work.
But without them, we wouldn’t be able to power the devices we rely on every day. With the proper care and maintenance, you can get years of use out of your rechargeable batteries.
Minerals Used in Electric Car Batteries
Electric car batteries are one of the most important components of an electric vehicle. Without a battery, an electric car would not be able to function. There are many different types of minerals used in electric car batteries, each with its own unique properties and benefits.
The most common type of mineral used in electric car batteries is lithium. Lithium is a soft, silver-white metal that is highly reactive. It is found in many rocks and minerals, including spodumene, lepidolite, and petalite.
Lithium has high electrical conductivity and is lightweight, making it ideal for use in batteries. Other minerals used in electric car batteries include cadmium, nickel, cobalt, manganese, and iron. These minerals are often combined with lithium to create battery cathodes that are more durable and have a longer lifespan.
Electric car batteries typically contain several thousand cells connected together to create the desired voltage output. Minerals play an essential role in the functionality of electric car batteries. without these key ingredients, electric vehicles would not be possible!
Why is Lithium Used in Batteries?
Lithium is the lightest metal and has the greatest electrochemical potential. This makes it an ideal material for batteries, which need to be lightweight and have a high voltage. Lithium batteries are used in many electronic devices, including cell phones, laptops, and electric cars. Lithium batteries have a number of advantages over other types of batteries. Here are some benefits of it:
| 1 | They can be discharged and recharged very quickly |
| 2 | they have a high energy density (meaning they can store more energy per unit weight than other types of batteries) |
| 3 | they are not susceptible to memory effect ( meaning they do not lose capacity over time if only partially discharged before being recharged) |
However, lithium batteries also have some drawbacks. They are expensive to produce, and their performance can be adversely affected by extreme temperatures.
Minerals in Batteries
Batteries are devices that store and release electricity. The most common type of battery is the lead-acid battery, which contains lead and sulfuric acid. Other types of batteries include lithium-ion, nickel-cadmium, and nickel-metal hydride.
Batteries convert chemical energy into electrical energy. This process is called electrolysis. During electrolysis, electrons are transferred from one electrode to another through an electrolyte solution.
The electrodes in a battery are made of different materials that have different abilities to attract and release electrons. The material with the greatest ability to attract electrons is called the cathode, while the material with the least ability to attract electrons is called the anode. The cathode is usually made of a metal oxide or hydroxide, while the anode is typically made of carbon or some other conductive material.
Lead-acid batteries use lead dioxide as the cathode and metallic lead as the anode. Lithium-ion batteries use lithium cobalt oxide as the cathode and graphite as the anode. Nickel-cadmium batteries use nickel hydroxide as the cathode and cadmium as the anode.
Nickel-metal hydride batteries use nickel oxide hydroxide as the cathode and a metal alloy as the anode. When a battery is connected to a circuit, ions flow from one electrode to another through the electrolyte solution until they reach equilibrium (a state in which there is no net flow of ions). This creates a potential difference between the two electrodes, which can be used to power electrical devices such as flashlights or computers.
What Minerals are Used in Solar Panels?
Solar panels are made up of many different materials (like glass), but the most important component is silicon.
Solar panels convert sunlight into electricity by using a process called the photovoltaic effect. When sunlight hits a solar panel, it knocks electrons loose from the atoms in the silicon.
Solar panels can charge without direct sunlight, but they are not as efficient as when they are in direct sunlight. you have to know that most solar panels are designed to withstand rain and other weather conditions, but it is still important to take steps to protect them.
Solar panels convert sunlight into electricity by using a process called the photovoltaic effect. When sunlight hits a solar panel, it knocks electrons loose from the atoms in the silicon.
These free electrons flow through the material to metal contacts on the sides of the cell, creating an electrical current. Other minerals used in solar panels include cadmium and tellurium. Cadmium is used in solar cells as a semiconductor, and tellurium helps create an electrical field within solar cells.
How is Raw Lithium Transported?
Raw lithium is transported in a number of ways, depending on the form in which it is found. In its natural state, lithium is found in minerals such as spodumene and petalite. Lithium can also be produced synthetically from other chemical elements.
Lithium ore must be mined and concentrated before it can be used commercially. Once concentrated, the ore is typically converted into lithium carbonate or lithium chloride, both of which are white powders that are relatively easy to handle and transport. Lithium carbonate is the most commonly used form of lithium, due to its high purity and stability.
It is usually shipped in 25kg bags or 1-tonne bulk bags. Lithium chloride is another popular form of lithium, although it is slightly more reactive than carbonate and therefore requires more careful handling during transportation. Once shipped to industrial users, raw lithium must be further processed before it can be used in end products such as batteries or lubricants.
Where is Lithium Found?
Lithium is a chemical element with the symbol Li and atomic number 3. It is a soft, silver-white metal belonging to the alkali metal group of elements. Lithium has a low density, is the lightest of all metals, and has the lowest melting point.
Lithium occurs in a number of minerals, but due to its solubility as an ion it is present in much greater concentrations in seawater and other lithium-bearing brines. The largest producers of lithium are Australia, Chile, and China. Other countries with significant reserves include Argentina, Bolivia, and Zimbabwe.
The main commercial source of lithium is the mineral spodumene (Lil(SiO3)2). Lithium production from spodumene is usually concentrated in Australia and China. Other minerals that contain lithium include lepidolite ((K, Li)Al3(Al, Si)4O10(F, OH)2), petalite ((Li-Al)4Si4O12), amblygonite (Li AlPO4), pollucite (CsAl Si2O6), and eucryptite (Li Al Si O4).
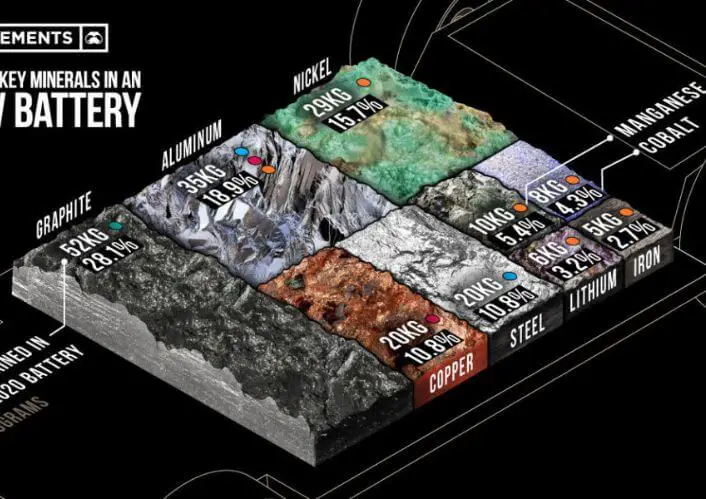
FAQs
What Minerals are Used to Make Lithium Batteries?
Lithium batteries are one of the most popular types of batteries on the market today. They are used in a wide variety of applications, from cell phones to laptops to electric cars. Lithium batteries get their name from the fact that they use lithium metal as one of their electrodes.
Lithium is a soft, white metal that is highly reactive. It is found in nature only in trace amounts, so it must be isolated from other materials in order to be used in batteries. The process of isolating lithium usually involves extracting it from minerals like spodumene or petalite.
Once isolated, the lithium metal can then be used as an electrode in batteries.
Lithium batteries have a number of advantages over other types of batteries. They are lightweight, have a high energy density, and can be discharged and recharged very quickly.
Additionally, they are relatively safe and stable compared to other battery chemistries.
Despite these advantages, there are also some drawbacks to using lithium batteries. One major issue is that they can be quite expensive to manufacture due to the cost of isolation and processing required for the lithium metal electrode.
Additionally, they require special handling and care when recycling due to the potential hazards posed by the reactive lithium metal.
Where Do the Minerals for Lithium Batteries Come From?
Lithium is a soft, silver-white metal that is part of the alkali metal group. It is the lightest metal on Earth and has the lowest density of all metals. Lithium does not occur naturally in its elemental form; instead, it is found in various minerals, such as spodumene, lepidolite, and zabuyelite.
The majority of lithium production takes place in Chile, Australia, and Argentina. These countries are home to large reserves of lithium-rich minerals, such as spodumene and brine. Chile alone produces about one-third of the world’s lithium supply.
Once extracted from the ground, lithium goes through a process of refinement to be used in batteries. First, the mineral ore is crushed and dissolved in sulfuric acid or another solvent. This solution is then filtered and treated with chemicals that cause the lithium to precipitate out as a white solid.
The solid is then dried and calcined (heated to a high temperature) to create pure lithium carbonate or other compounds used in batteries.
What is the Main Mineral in Lithium?
Lithium is a soft, silver-white metal that is part of the alkali metal group. It is the lightest metal on Earth and has the highest atomic number of any element that occurs naturally in the Universe. Lithium’s low reactivity means it does not corrode in air or water, making it ideal for use in batteries and other high-tech applications.
The main mineral in lithium is spodumene, which can be found in pegmatite deposits around the world.
What are the Main Ingredients in a Lithium Battery?
Lithium batteries are one of the most popular types of batteries on the market today. They are used in a wide variety of devices, from cell phones to laptops. But what exactly are they made of?
The main ingredient in a lithium battery is, not surprisingly, lithium. Lithium is a metal that is found in nature, but it can also be produced synthetically. It’s relatively lightweight and has a very high energy density, making it ideal for use in batteries.
In addition to lithium, batteries also contain other materials like electrolytes and electrodes. The electrolyte helps to keep the charge flowing between the electrodes, while the electrodes actually store and release the electrical energy. Lithium batteries have a number of advantages over other types of batteries.
They’re lightweight, long-lasting, and able to hold a large amount of power relative to their size. However, they can also be expensive and dangerous if not handled properly.
Conclusion
Lithium batteries are used in a variety of devices, from cell phones to laptops. But what minerals are used in lithium batteries? The three main minerals used in lithium batteries are cobalt, nickel, and manganese.
Cobalt is found in the cathode, or positive electrode, of the battery. Nickel is found in the anode or negative electrode. Manganese is found in the electrolyte, which helps to keep the battery stable.
These minerals are important because they help to make the battery more efficient. For example, cobalt helps to increase the capacity of the battery while nickel helps to improve its discharge rate. Manganese also helps to improve both the capacity and discharge rate of the battery.
Lithium batteries are an important part of our lives and will continue to be so for years to come. So it’s important to understand what minerals are used in them and why they’re so important!