A lead-acid battery is a type of rechargeable battery that uses a chemical reaction between lead and sulfuric acid to create electricity. The working principle of a lead-acid battery is based on the fact that lead and lead dioxide are both excellent electrical conductors. When these two materials are combined in an acidic solution, they form a series of interlocking plates that act as electrodes.
A car battery is a type of lead-acid battery. It is usually made up of six cells, each cell producing 2 volts for a total 12-volt battery (on average, a 12-volt battery weighs about 10 to 15 pounds.). The cells are connected in series meaning the negative terminal of one cell is connected to the positive terminal of the next cell.
This creates a circuit between all the cells and allows current to flow through them. The chemical reaction that takes place inside the cells produces electrons that flow from the negative to the positive terminal. This flow of electrons provides the power needed to run your car’s electrical accessories such as lights, radio, etc.
When you start your car, the starter motor requires a large amount of power which is supplied by the battery. Once your engine is running, your alternator takes over and provides power for your electrical accessories and recharges your battery at the same time.
Car Battery Specifications
Assuming you would like a blog post discussing car batteries and their specifications: A car battery is a lead-acid battery that supplies electric energy to an automobile. Usually, this refers to the starting battery in a vehicle as it is typically of a large capacity and provides a relatively high current during cranking.
The main purpose of a starter battery is to crank the engine, which then powers the alternator to charge the rest of the electrical system. A typical 12-volt automotive battery contains six cells connected in series; each cell has 2.1 volts for a total voltage of 12.6 volts at full charge. Most passenger cars have 12-volt batteries, while larger vehicles such as trucks and buses may have 24-volt systems.
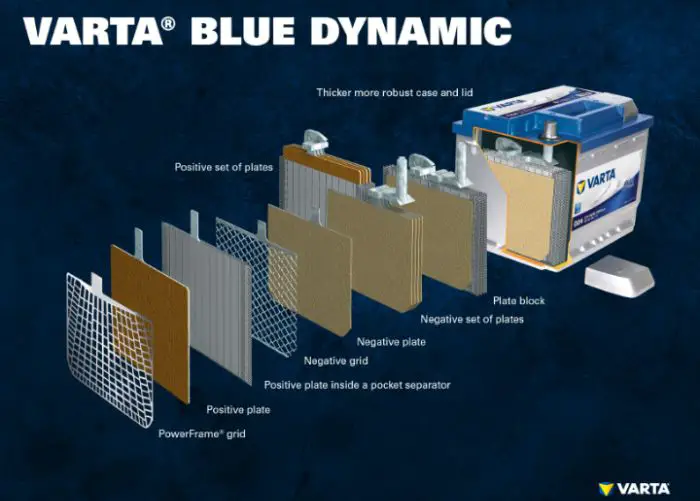
Batteries are made up of several individual cells housed in a single unit. Inside each cell are positive and negative electrodes (made from lead) separated by an electrolyte (usually sulfuric acid). When you connect your car’s battery terminals to an external power source, electrons flow from the negative terminal through the external circuit back to the positive terminal completing an electrical circuit.
The chemical reaction inside each cell produces electrons on the negative side and ions on the positive side. These ions attach themselves to molecules of water in the electrolyte producing hydrogen gas bubbles on the surface of the positive electrode. As more hydrogen gas bubbles form, they begin to interfere with electron flow reducing efficiency and causing a voltage drop across terminals until eventually no current flow at all if left unchecked.
Over time, this process will destroy a lead acid battery unless it is regularly recharged by running the engine or using an external charger. It’s important to know that they take a long time to charge.
How Does a Car Battery Charge?
A car battery is a 12-volt lead-acid battery that is used to start an engine. It is made up of six 2-volt cells connected in series. Each cell has a positive and negative plate separated by an electrolyte (sulfuric acid).
When the engine is running, the alternator produces electricity that flows through the charging system and charges the battery. The charging system has three main components: the alternator, the voltage regulator, and the battery. The alternator is a belt-driven device that converts mechanical energy into electrical energy. An electric fan uses electrical energy to create mechanical energy.
How fast does an alternator charge a battery? It depends on the capacity of the alternator and the condition of the battery. A healthy battery should be able to recharge in about an hour.
The voltage regulator regulates the amount of voltage that goes to the battery so that it doesn’t overcharge or undercharge it. The battery stores the energy produced by the alternator and provides it to the starter motor when needed. When you turn on your car, the starter motor uses electricity from the battery to turn over the engine and start it running.
Once started, your car’s engine runs off of gasoline or diesel fuel, not electricity from the battery. However, even while your car’s engine is running, some of its electrical systems still rely on power from the battery – for example, lights (including headlights), radio, GPS navigation system, etc. That’s why it’s important to keep your car’s battery charged. But it is not advisable to let a car battery charge overnight.

Car Battery Voltage
Most car batteries have a voltage of 12.6 volts. This is the amount of power that is needed to start most cars. The battery voltage can drop as low as 10 volts when the engine is off and will rise to 13.8 volts when the engine is running.
A car battery can last for many years if it is properly maintained. The average car battery lasts between two and five years.
How Car Battery Works Animation?
We all know that car batteries are an essential part of any vehicle. They provide the power needed to start the engine and keep it running. But how do car batteries work, exactly?
Here’s a helpful animation that shows how a typical car battery works: As you can see, the battery is made up of two lead plates (the positive and negative) separated by an electrolyte solution. When the engine is off, the lead plates are not in contact with each other.
However, when you turn on the ignition, a circuit is completed between the plates and electrons flow from the negative plate to the positive plate. This flow of electrons produces an electric current that powers your car’s engine. The battery will continue to produce electricity as long as there is a difference in voltage between the two lead plates.
However, over time, this voltage difference decreases as the lead plates become corroded by the electrolyte solution. When this happens, it’s time for a new battery!
What are the 4 Functions of a Car Battery?
A car battery is an important part of a car’s electrical system. It provides power to the starter motor, ignition system, and other accessories. The battery also helps stabilize voltage levels and provide electrical current when the engine is not running.
Here are four key functions of a car battery:
| Key function 1 | Starting the engine | The battery provides power to the starter motor, which is responsible for starting the engine. |
| Key function 2 | Igniting the spark plugs | The battery supplies electricity to the ignition system, which ignites the spark plugs and starts combustion in the engine cylinders. |
| Key function 3 | Running accessories | When the engine is off, the battery powers electrically-powered accessories like headlights, interior lights, and radios. |
| Key function 4 | Stabilizing voltage | The battery helps maintain stable voltage levels in the electrical system while the engine is running. This prevents damage to sensitive electronic components like computers and onboard diagnostics systems. |
How Does a Car Battery Die?
A car battery can die for a number of reasons. The most common cause of death for a car battery is simply aged. Batteries have a finite lifespan, and after a certain number of years (usually between 3-5), they will no longer be able to hold a charge.
Another common cause of battery death is exposure to extreme temperatures – both hot and cold. If your battery is regularly exposed to very high or low temperatures, it will shorten its lifespan considerably. Another factor that can contribute to battery death is the lack of maintenance.
If you don’t regularly check your battery’s fluid levels and clean the terminals, it will eventually lead to corrosion and damage that can kill the battery. Finally, if you frequently use your car for short trips instead of long ones, your battery may not get the chance to fully recharge, which can also lead to its eventual demise.
Quick Facts
How Does a Car Battery Work?
A car battery is a lead-acid battery that supplies electricity to a car. It is also known as a starter battery or SLI (starting, lighting, ignition) battery. A car battery has six cells that produce 2 volts each for a total of 12 volts.
The cells are made of lead and filled with an electrolyte solution of water and sulfuric acid. When the engine is running, the alternator charges the battery and provides power for the electrical system. When the engine is off, the battery provides power for the ignition system, lights, and other accessories.
If your car battery dies, you may be able to use your alternator to charge it back up again. However, it’s important to know that an alternator will not fully charge a dead battery.
The average life of a car battery is two and five years.
What Type of Battery is a Car Battery?
A car battery is a lead-acid battery, which means that it is made up of lead plates and acid. The lead plates are divided into two types: positive and negative. The positive plate is covered with a material called active mass, which helps to increase the surface area for the chemical reaction that takes place when the battery is charging.
The negative plate is made up of Lead dioxide, which reacts with the acid to create electrons. These electrons flow from the negative plate to the positive plate during discharge, creating electricity. The main types of car batteries are starter batteries, deep cycle batteries, and dual-purpose batteries.
Starter batteries are designed to start engines, while deep cycle batteries are designed to power accessories like lights or radios for long periods of time without recharging. Dual-purpose batteries can be used for both starting engines and powering accessories.
What is Inside of a Car Battery?
Inside a car battery, there are six cells connected in series. The positive terminal of the first cell is connected to the negative terminal of the second cell, and so on. Each cell has lead plates (the positive plate is covered with lead dioxide, while the negative plate is covered with spongy lead) submerged in a solution of sulfuric acid and water.
When the engine is running, a chemical reaction takes place between lead and sulfuric acid, which releases electrons. These electrons flow through the external circuit to the negative terminal of the next cell in line. When they reach the end of the line, they flow back to the positive terminal of the first cell, completing the circuit.
This flow of electrons provides power to run your car’s electrical components.
Conclusion
How a car battery works is actually pretty simple. It’s made up of two lead plates that are submerged in an electrolyte solution. When the engine is running, the alternator produces an electrical current that flows through the leads and causes a chemical reaction.
This reaction creates electrons on one plate and ions on the other, which generates a voltage between the two plates. The higher the voltage, the more powerful the electrical current flowing through the battery will be.