Energy is a fundamental concept in physics and it refers to the ability to do work. The three laws of conservation of energy state that energy cannot be created or destroyed, only converted from one form to another. The first law is the law of conservation of mass-energy, which states that the total amount of energy in a closed system remains constant over time.
The second law is the law of conservation of momentum, which states that the momentum of a system must remain constant if there are no external forces acting on it. The third law is the law of conservation of angular momentum, which states that the angular momentum of a system must remain constant if there are no external torques acting on it.
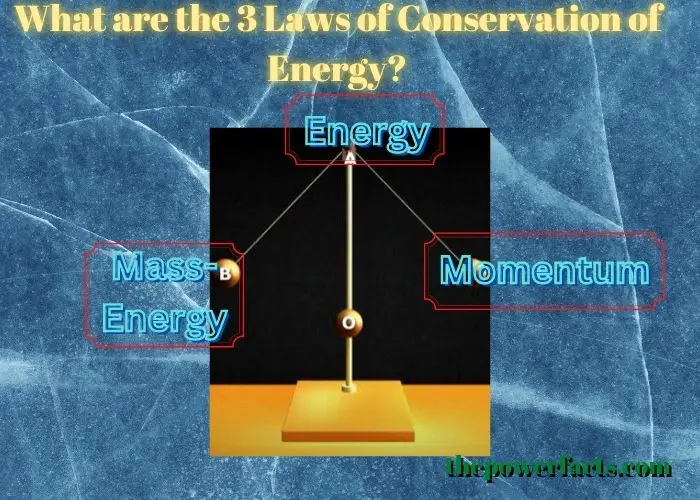
The three laws of conservation of energy are as follows:
1) Energy cannot be created or destroyed, only transformed from one form to another.
2) The total amount of energy in the universe is constant.
3) Energy can neither be created nor destroyed; it can only be converted from one form to another. These three laws help to explain many phenomena in the natural world, and they have important implications for our understanding of how energy works. For example, the first law tells us that we cannot create new energy, but we can transform existing energy into new forms.
This is why we have to conserve energy – because it’s a finite resource that we cannot replace. The second law explains why some forms of energy are more useful than others. It also tells us that when we use energy, it doesn’t disappear – it just changes form.
For example, when you burn fossil fuels like coal or oil, the chemical energy is released and converted into heat and light. But this heat and light eventually dissipate into the surroundings, and the overall amount of energy in the universe remains constant. Finally, the third law helps us understand why certain processes are irreversible.
Once energy has been converted from one form to another, it cannot be turned back into its original form again. This is why things like burning fossil fuels or radioactive decay are considered irreversible processes – once the conversion has happened, there’s no going back!
State the Law of Conservation of Energy With Example
The Law of Conservation of Energy is the fundamental law of physics that states that energy cannot be created or destroyed, but can only be converted from one form to another. The law is a direct consequence of the fact that the total mass-energy of the universe is constant. The principle was first put forth by German physicist Hermann von Helmholtz in 1847, who stated that “the energy of the world is a constant; it can neither be increased nor diminished.”
In other words, the total amount of energy in the universe remains constant, even as it changes form. For example, when a ball is dropped and hits the ground, its potential energy (due to its height) is converted into kinetic energy (motion). However, the total amount of energy in the system (ball + earth) remains unchanged. When you use solar panel you need to know how much Energy Does a 7Kw Solar System Produce.
State the Law of Conservation of Energy
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a fundamental concept of physics along with the laws of conservation of mass and conservation of momentum. The total energy of a system can take many different forms, including kinetic energy, thermal energy, electromagnetic radiation, gravitational potential energy, and rest mass energy. You have to select what form last in solar system.
The sum of these energies always remains constant for an isolated system. However, in order to conserve energy within a closed but not necessarily isolated system (such as within Earth’s atmosphere), it is necessary for heat to flow from hotter regions to cooler ones until thermal equilibrium is reached. In other words, there can be no net flow of energy into or out of the system; it can only change form.
One way to understand why the law exists is to consider two hypothetical objects: a ball at rest on a table top and a ball rolling across the floor. If we assume that both balls are made out of the same material and have the same size then they must have equal amounts of force required to stop them (i.e., their Momentum). Now if we imagine that someone comes along and pushes on the ball on the floor so that it speeds up, according to Newton’s second law of motion (Force = Mass × Acceleration), the force required to stop this ball will also increase linearly with its speed – meaning that its Momentum is increasing at twice the rate as its force (because Force = Mass × Change in Velocity/Time).
So if we were somehow able to transfer the extra Momentum from the ball on the floor into our stationary ball on top of the table then by Newton’s third law of motion every action would have an equal and opposite reaction– resulting in both balls having exactly the same Momentum and moving away from each other at equal speeds!
State the Law of Conservation of Energy Class 9
The law of conservation of energy is one of the most important laws in physics. It states that energy can neither be created nor destroyed. This means that the total amount of energy in the universe is always constant.
The law of conservation of energy is also known as the first law of thermodynamics.
The law of conservation of energy can be applied to any system, whether it’s a closed system like the universe or an open system like a car engine. In a closed system, the total amount of energy stays constant because there’s no way for energy to come into or leave the system.
In an open system, some energy may be converted from one form to another (for example, heat may be converted to work), but the total amount ofenergy in the system remains constant.
What Does the Law of Conservation of Mechanical Energy State?
In physics, the law of conservation of mechanical energy states that the total mechanical energy in an isolated system remains constant—it is said to be conserved over time. This law is a direct consequence of Newton’s laws of motion and energy. The sum of kinetic energy and potential energy in any closed system always remains constant.
The law applies to both elastic collisions and inelastic collisions. In an elastic collision, both kinetic energy and momentum are conserved. In an inelastic collision, only kinetic energy is conserved—momentum is not necessarily conserved because some of the kinetic energy may be converted into other forms such as heat or sound.
Who Discovered the Law of Conservation of Energy?
In 1845, German physician Julius Robert von Mayer discovered the law of conservation of energy while studying human metabolism. Von Mayer realized that the body’s metabolic processes were converting the chemical energy in food into mechanical work and heat. He proposed that these conversions were all governed by the laws of thermodynamics.
His work was later expanded upon by other scientists, including James Joule and Lord Kelvin.
If Energy Cannot Be Created Or Destroyed, Where Did It Come from?
In the early universe, there was only energy. No matter, no photons, no particles whatsoever- just energy. And this energy was not static or still in any way; it was constantly moving and shifting and changing.
So where did this energy come from?
The answer, quite simply, is that we don’t know. Energy is a fundamental part of our universe, and it has been here since the beginning.
It is possible that it has always existed, in some form or another. Or perhaps it was created at the moment of the Big Bang itself. We may never know for sure.
What we do know is that energy cannot be created or destroyed. It can only change forms. So whatever its source may be, energy will always be a part of our universe.
Energy Cannot Be Created Or Destroyed Which Law
The law of conservation of energy states that energy cannot be created or destroyed. This means that the total amount of energy in the universe is constant. Energy can, however, be converted from one form to another.
For example, it can be converted from heat to light or from mechanical to electrical energy.
This law is one of the most important laws in physics. It helps us understand many natural phenomena, such as why a hot object cools down over time.
It also has important applications in engineering and technology.
Example of Law of Conservation of Energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a direct consequence of the fact that the laws of physics are time-reversible. The total energy includes both kinetic energy, which is associated with the motion of objects, and potential energy, which results from forces acting on those objects.
What are the 3 Law of Conservation?
The three laws of conservation are:
| 1 | The law of conservation of energy | Energy can neither be created nor destroyed, but it can be transformed from one form to another. |
| 2 | The law of conservation of momentum | Momentum is conserved in a closed system. |
| 3 | The law of conservation of mass | Mass is conserved in a closed system. |
What are the 2 Law of Conservation of Energy?

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a consequence of the fact that energy cannot be created or destroyed, although it can be converted into different forms. The total amount of energy in a system can therefore only change if energy is transferred into or out of the system.
For example, when a ball falls to the ground and bounces back up again, its potential energy has been converted into kinetic energy and then back into potential energy again. However, no net loss or gain in overall energy has occurred, because the sum of these two types were always equal to each other. There are two forms of mechanical Energy: Potential Energy and Kinetic Energy.
Potential Energy is stored within an object due to its position relative to another object (gravitational potential), its condition (elastic potential), or by electric charge (electrostatic potential). An everyday example would be holding a book above your head; work must be done against gravity to maintain this position. If you were then to release the book, it would fall towards the ground and as it gains speed, its gravitational potential decreases while its kinetic increases until it reaches terminal velocity where these energies reach equilibrium.
Kinetic Energy on the other hand is associated with motion; an object with KE possesses mass and is moving. It could be argued that anything which isn’t at rest has some form of KE whether natural movement like tides/winds or manmade like cars/planes etc.. The faster something moves, generally speaking, the more KE it will have – think about how much effort it takes you to walk up a flight stairs versus running full pelt up them!
When calculating KE you must consider both mass (m) and velocity (v): KE = 1/2 mv^2 . As you can see from this equation, doubling either mass OR velocity will result in quadrupling the kinetic energy!
What are the 4 Laws of Conservation?
The laws of conservation are four fundamental principles that underpin all scientific inquiry. They state that energy, momentum, mass, and charge are conserved in any physical system. These laws help to explain the behavior of matter and energy on both the macroscopic and microscopic scales.
The Law of Conservation
The law of conservation of energy states that energy can neither be created nor destroyed. This means that the total amount of energy in a closed system is always constant. The law of conservation of momentum states that momentum is also conserved in a closed system.
Momentum
This means that the total momentum of all objects in a system will remain constant unless an external force acts upon the system.
The Law of Conservation of Mass
The law of conservation of mass states that mass can neither be created nor destroyed. This means that the total amount of mass in a closed system is always constant.
The Law of Conservation of Charge
The law of conservation of charge states that charge can neither be created nor destroyed. This means that the total amount of electrical charge in a closed system is always constant. For solar system you need to upgrade your electrical panel. These laws are some of the most important principles governing our understanding of the natural world.
Without them, we would not be able to make predictions about how matter and energy will behave under various circumstances.
What are the Three Examples of Conservation of Energy?
The three examples of conservation of energy are:
| Number | Conservation of energy |
| 1 | Energy can neither be created nor destroyed, only transformed from one form to another. For example, when a hydroelectric dam generates electricity, the water’s potential energy is converted into kinetic energy, and then into electrical energy. |
| 2 | The total amount of energy in an isolated system (one that is not interacting with its surroundings) remains constant over time. This means that if there is more energy in one part of the system, there must be less elsewhere. For example, if a roller coaster starts at the top of a hill with high potential energy, it will have less potential energy at the bottom as it has transferred some to kinetic energy. However, the total amount ofenergy (potential plus kinetic) stays the same. |
| 3 | Energy can be neither created nor destroyed, but it can be converted from one form to another. For example, when coal is burned to generate electricity, the chemical energy in the coal is converted into heat and light (thermal and electromagnetic radiation). |
Conclusion
In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. This law is a consequence of the fact that the laws of physics are time-reversible. The total energy includes both kinetic energy, which is associated with the motion of particles, and potential energy, which reflects the force between particles.
Related Posts: